Fluorescence, Phosphorescence, Chemiluminescence
Fluorescence, Phosphorescence, Chemiluminescence are types of luminescence. In each of these processes, light is emitted: For Flurescence and Phosphorescence after the absorption of light. For the chemiluminescence as the result of a chemical reaction.
Underground-Minigolf

Today, brilliant colours that only appear in UV light, are part of everybody´s everyday experience. Usually photons of higher energy are turned into photons of lower energy. In a fascinating experiment this video shows that this can also work vice versa, and gives a clear and convincing explanation.
Photoluminescence - Color by Emission of Light (German Voice-Over)

Whether it is the white T-shirt that glows in the disco or the digits of the clock that glow in the dark after irradiation with light, most people know the phenomena of fluorescence and phosphorescence from everyday life. What many do not know is that modern lamps such as fluorescent tubes, energy-saving lamps and LEDs make use of these two phenomena.This educational film examines the causes of fluorescence and phosphorescence. For this purpose, experiments are carried out in the laboratory and the processes are explained on an atomic level. In order to give the viewer an insight into the practical usability, the topic of modern lamps will be examined in more detail.
Fluorescence of Esculin (The Crying Chestnut Branch)
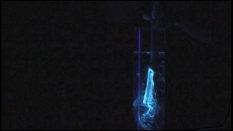
A short video showing a blue-white glowing substance, esculin, flowing out of the incision into a chestnut branch under irradiation with UV light (λ = 365 nm). The substance spreads within the water.
Preparation of a Phosphorescent Sample

The video shows how a phosphorescent sample can be prepared using tartaric acid and esculin. The irradiation of the sample with UV-light with the still warm sample is demonstrated as well after the sample has cooled down to room temperature.
Duration of the Phosphorescence of Fluorescein-Boric Acid-Samples at Different Temperatures
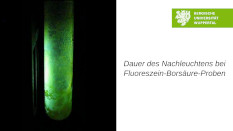
This video demonstrates that the duration of the phosphorescene of fluoresceine-boric acid-samples is dependent on the temperature. Three equal samples at different temperatures (− 70 °C, 20 °C und 80 °C ) are shown during irradiation and the duration of their phosphorescence afterwards.
Duration and Colors of the Phosphorescence of H-Acid-Boric Acid-Samples

This video shows the increased duration of phosphorescence directly after switching of the light source with H acid-Boric Acid-Samples. During this process the difference in color between fluorescence and phosphorescence becomes apparent.
Extending the Duration of Phosphorescence by Cooling

This video shows how the phosphorescence of an esculin in genatine sample can be significantly extended by cooling it with liquid nitrogen.
Photoprotection of Chloropyll by β-Carotene
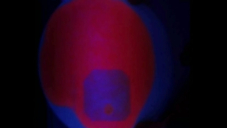
This Video demonstrates an experiment showing the photoprotection of chlorophyll by β-Carotene. For this pumpkin seed oil is mixed 1:1 with acetone and applied to a filter paper. Under irradiation with UV light the chlorophyll shows an intensive red fluorescence.
After applying β-carotene solution in heptane to a small point on the filter paper, the fluorescence of the chlorophyll is reduced. In the next step the filter paper is irradiated in a large area around the spot with the β-carotene solution. The fluorescence of the chlorophyll in this area is almost completely gone, only the spot that had β-carotene applied to it shows the same fluorescence as before the filter paper was irradiated.
Cold Incandescence - Chemoluminescence of Luminol (new)
![Cold Incandescence - Chemiluminescence of Luminol [new] Cold Incandescence - Chemiluminescence of Luminol [new]](/fileadmin/_processed_/7/2/csm_video-weissglut-neu_6f7723308f.png)
Potassium hydroxide is wetted using DMSO, and a spatula tip luminol is added.
This results in chemoluminescence after a short while. How nitrogen and oxygen impact the reaction that generates the luminescence, is demonstrated after the base experiment.
Cold Incandescence - Chemoluminescence of Luminol

This video shows the Cold Incandescence.
Extinguishing the Cold Incandescence

This video shows how the chemoluminescence of luminol begins to fade after introducing nitrogen into the flask.
Reigniting the Cold Incandescence

This video shows how the "extinguished" chemoluminescence of luminol can be reignited with oxygen.
