 |
Hydrophobic Properties of Silicone Fluids/Variant
B |
 |
  1
Materials, Chemicals, Time Needed 1
Materials, Chemicals, Time Needed |
 |
|
Each sample takes
less than 5 minutes.
|
 |
  2
Procedure and Observations 2
Procedure and Observations |
 |
| Uniformly coat part of the various surfaces
with silicone fluid. Apply a drop of water to both the siliconized
and the untreated surfaces. |
 |
 |
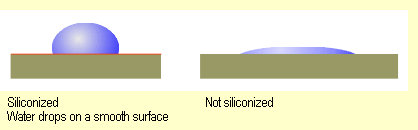 |
The water droplets on the siliconized
surfaces are comparatively spherical and do not run off.
When viewed on smooth surfaces from the side, they are
much taller. They are slowly absorbed by paper tissues
and cotton fabric. |
|
| In contrast, the water drops
on the untreated surfaces run off more extensively and also adhere
better to them. Cotton fabric and paper tissues absorb the water
droplets immediately. |
 |
  3
Discussion of Results 3
Discussion of Results
|
 |
 |
 |
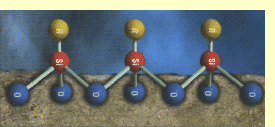
The experiment reveals a marked increase in contact
angle for non-absorbent smooth surfaces. The contact
angle, θ, is a measure of
a substance’s water repellency and is illustrated
in the adjacent diagram.
The molecules of the silicone
fluid spontaneously align themselves on the surface
such that the methyl groups
stick out from the surface. They create the water-repellent
effect. In the diagram below, the methyl groups are
represented by R.
|
 |
|
| The diagram is taken from the WACKER WERK + WIRKEN
magazine quoted in the References. |
 |
 |
  4
Tips and Comments 4
Tips and Comments
|
 |
- The silicone fluid can be
baked into glass and then rubbed off with a paper towel
so that the coating is almost invisible. Even then, the water-repellent
effect can still be observed. Baking can be done by dry
heating
the silicone-treated glass at over 100 °C for 10 to
15 minutes.
- For more information on water-repellent or hydrophobic
properties, see also "Variant
A" of
this experiment and the experiment "Siliconized
filter paper."
- This experiment and the ones mentioned
above are safe and may be performed without reservation
by the pupils. They are suitable
for demonstrating the relationship between particle structure
and material properties. This CD and the references listed
below contain substantial amounts of information that
may be used in
chemistry lessons.
|
 |
  5
Supplementary Information 5
Supplementary Information
|
 |
See also 5 Supplementary Information
on Variant A of this
experiment. |
 |
  6
References 6
References |
 |
- M. Tausch, M. von Wachtendonk (editors), CHEMIE S II, STOFF-FORMEL-UMWELT,
C.C. Buchner, Bamberg (1993), (1998)
- M. Tausch, M. von Wachtendonk (editors), STOFF-CHEMIE S
I, FORMEL-UMWELT, C.C. Buchner, Bamberg (1996), (1997)
- M. Tausch, M. von Wachtendonk (editors), CHEMIE 2000+,
C.C. Buchner, Bamberg (2001)
- WACKER Werk+Wirken, Zeitschrift der Wacker Chemie AG,
München, 48 (6), 22 (1997)
|
 |
 |
|