 |
Hydrolysis of Chloromethylsilanes |
 |
  1
Materials, Chemicals, Time Needed 1
Materials, Chemicals, Time Needed |
 |
- Test-tube rack
- 6 test tubes
- 3 pipets (2 ml)
- Pipet bulb
- Graduated cylinder (20 ml)
- Dropping pipet
- Glass rod
- Suction bottle
- Büchner funnel
- Filter adapter
- Water-jet pump
- Chlorotrimethylsilane (M), C, F
- Dichlorodimethylsilane (D), Xi, F
- Trichloromethylsilane (T), Xi, F
- Universal indicator paper
- Filter paper
The whole experiment takes about 15 minutes, with
5 minutes for preparation, 3 minutes for
hydrolysis and 3 minutes for the pH test. The remaining
time is needed for preparing
the
silicone resin powder.
|
 |
  2
Procedure and Observations 2
Procedure and Observations |
 |
As chloromethylsilanes
hydrolyze very easily to release hydrogen chloride, perform
the experiment in a fume cupboard.
Wear safety glasses, rubber gloves and laboratory coat.
- In the fume cupboard, fill three
test tubes with 6 ml, 12 ml and 18 ml water. To three other
test tubes, add 2 ml each of the different chloromethylsilanes.
Proceed as follows:
- Add 6 ml water to the test tube containing chlorotrimethylsilane
and observe the reaction. The mixture initially turns hazy
and then two liquid phases form.
- Add 12 ml water to the test
tube containing dichlorodimethylsilane and observe the
reaction. A vigorous reaction occurs, and a
gas is evolved. The lower, initially hazy phase gradually
turns clear, just like the upper phase.
- Add 18 ml water to the
test tube containing trichloromethylsilane and observe
the reaction. Strong evanescence occurs, a gas
is evolved and a solid forms that sticks firmly to
the walls of the test-tube and is difficult to remove.
|
|
 |
  3
Discussion of Results 3
Discussion of Results
|
 |
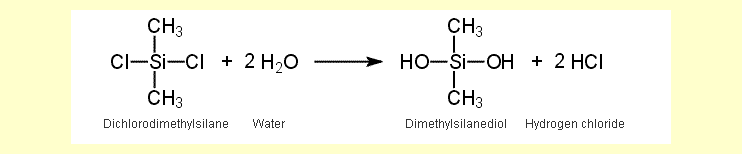
None of the three chloromethylsilanes
is resistant to water and all three hydrolyze to methylsilanols
and hydrogen chloride. The equation for the reaction of dichlorodimethylsilane
is shown here as an example:
The methylsilanols condense immediately to larger molecules. In the case
of the monofunction trimethylsilanol, condensation ceases with the formation
of hexamethyldisiloxane, a silicon compound whose molecules have a structure
similar to that of an ether. Hexamethyldisiloxane is a low-viscosity liquid
with a fairly high vapor pressure.
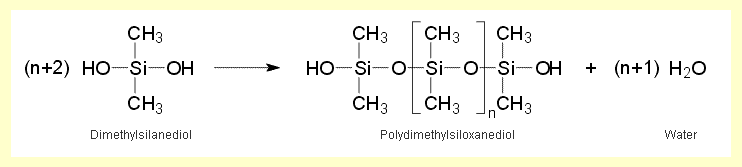
Dimethylsilanediol condenses to larger molecules that may be either chain-like
or cyclic. These are polydimethylsiloxanes, better known as silicones.
The longer the chains, the more viscous is the product. The formation of
chain-like silicone macromolecules is shown below:
Methylsilanetriol, the trimethylsilane hydrolysis product, condenses very
rapidly to extensively crosslinked silicone macromolecules. |
 |
  4
Tips and Comments 4
Tips and Comments
|
 |
- The hydrolysis of trichloromethylsilane
is one of the few reactions in which a macromolecular material
can be produced so easily, quickly and convincingly. Experienced
experimenters can use this example as a demonstration.
- The hydrolysis
of dichlorodimethylsilane and the spontaneous condensation
of the product are also suitable for demonstration
purposes, both in experimental lectures and in school lessons.
|
 |
  5
Supplementary Information 5
Supplementary Information
|
 |
| These experiments complement
the information on nucleophilic substitution and industrial production
of silicones which is contained in the textbook indicated below. |
 |
  6
References 6
References |
 |
| M. Tausch, M. von Wachtendonk
(editors), CHEMIE S II, STOFF-FORMEL-UMWELT, C.C. Buchner, Bamberg
(1993), (1998), S. 337f., S. 345f., S. 340 |
 |
 |
|