 |
Hydrophobic Properties of Silicone Fluids/Variant
A |
 |
  1
Materials, Chemicals, Time Needed 1
Materials, Chemicals, Time Needed |
 |
- Dropping pipet
- Various clean surfaces:
- Glass
- Copper panel
- Beer mat
- Paper tissue
- Polyethylene panel
- Polyvinyl chloride panel
- Wooden panel
- Candle
- Glycerol
- Silicone Fluid
AK 5000 from WACKER's Experimental Kit
- Methylene blue
The experiment will take around 10 minutes for
each surface.
|
 |
  2
Procedure and Observations 2
Procedure and Observations |
 |
| Uniformly coat part of the various
surfaces with the respective oils. Then apply a layer of candle
wax to a piece of card. Apply a drop of water to both the treated
and the untreated surfaces.
As may be seen from the photos, the water drops on the siliconized
surfaces and the card coated with candle wax form fairly spherical
droplets and do not spread out. Viewed from the side, the drops
on the treated surfaces are much higher than those on the untreated
surfaces.
|
 |
 |
 |
 |
Untreated
glass surface
|
Siliconized
glass surface
|
Glass surface treated
with glycerol
|
Card coated with
candle wax |
|
  The
water on the siliconized surfaces is absorbed much more slowly
than that on the untreated surfaces. It is not possible to
calculate the contact angle with the naked eye. The
water on the siliconized surfaces is absorbed much more slowly
than that on the untreated surfaces. It is not possible to
calculate the contact angle with the naked eye.
The water drops
on the surfaces treated with glycerol spread out extensively
and are quickly absorbed. Thus, the water-repellency
of a copper panel that has been thoroughly cleaned with ethyl
alcohol is substantially reduced by glycerol. In contrast,
the silicone fluid actually increases the water repellency.
|
 |
 |
 |
Untreated
copper panel
|
Copper panel treated with glycerol
|
Copper panel treated
with silicone fluid |
|
 |
  3
Discussion of Results 3
Discussion of Results
|
 |
| As the results show,
the surfaces treated with silicone fluid have much better water
repellency than the untreated surfaces. This is reflected in
the marked increase in the contact angle. The silicone molecules
spontaneously arrange themselves on surfaces that have polar
centers, e.g. glass, building materials and textiles, in such
a way that the methyl groups protrude from the surface. They
are responsible for the hydrophobic (water-repellent) effect
of the treated surfaces. |
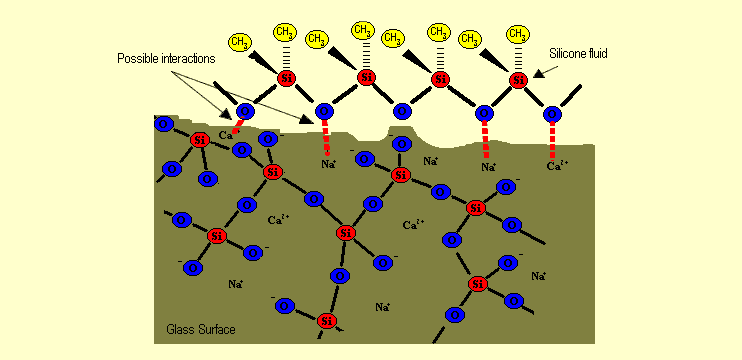 |
  The
structural similarity of the silicone fluids and the surfaces
of the glass, building materials and textiles are the reason
why the fluids adhere well to the surfaces. In the case of glass,
electrostatic forces of attraction cause bonds to form between
the negatively charged oxygen atoms of the silicone molecules
and cations within the glass. With paper, the polar oxygen atoms
form hydrogen bonds with the –OH groups in the cellulose
(see both diagrams). The
structural similarity of the silicone fluids and the surfaces
of the glass, building materials and textiles are the reason
why the fluids adhere well to the surfaces. In the case of glass,
electrostatic forces of attraction cause bonds to form between
the negatively charged oxygen atoms of the silicone molecules
and cations within the glass. With paper, the polar oxygen atoms
form hydrogen bonds with the –OH groups in the cellulose
(see both diagrams). |
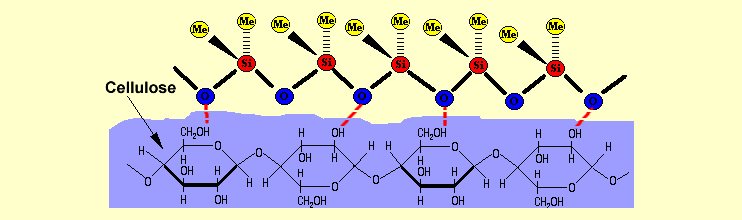 |
 Interactions
between cellulose and silicone fluid Interactions
between cellulose and silicone fluid |
 |
  The
card coated with candle wax is water repellent because the candle
wax is actually solid paraffin wax, i.e. it is a mixture of saturated
aliphatic hydrocarbons. These are nonpolar molecules that, like
the methyl groups in the silicone fluid, have hydrophobic properties.
They interact very weakly with the water molecules and so the
water stays in the drop and does not wet the paraffin wax. The
card coated with candle wax is water repellent because the candle
wax is actually solid paraffin wax, i.e. it is a mixture of saturated
aliphatic hydrocarbons. These are nonpolar molecules that, like
the methyl groups in the silicone fluid, have hydrophobic properties.
They interact very weakly with the water molecules and so the
water stays in the drop and does not wet the paraffin wax.
Paraffin wax is considered to be the most hydrophobic water-repellent
agent. However, paraffin waxes do not adhere as well as silicone
fluids to surfaces made of stone, glass or paper, and can be
scratched off.
Another major advantage over paraffin waxes is that silicone
films have a very low surface tension, a fact which makes them
highly spreadable and which enables hydrophobic effects to
be achieved with much smaller quantities. Unlike paraffin waxes,
silicone fluids have hardly effect on surface properties, such
as their appearance and “ability to breathe.”
The
increase observed in the hydrophilic properties of surfaces
treated with glycerol may be explained in terms of the structure
of glycerol. The glycerol molecule C3H5(OH)3 has three hydroxyl groups that form hydrogen bonds with water
molecules. Glycerol can therefore be mixed in any proportion
with water. |
 |
 |
  Extension
of experiment Extension
of experiment
With the aid of a camera, a computer and image-processing software, it
is possible to calculate the contact angle, as shown on the right. |
 |
| The following results were obtained
in a reference experiment: |
 |
| Type of
surface |
Contact
angle for water droplet |
Literature
value |
Siliconized glass surface
|
Approx. 103° |
100-110° |
| Copper panel |
Approx. 74 ° |
|
| Polyethylene |
Approx. 95° |
Approx. 95° |
| Paraffin waxes |
Approx. 103° |
Approx. 105° |
| Glass (degreased) |
Approx. 35° |
0° |
|
 |
| Aside from the results
for glass, the results agree well with the literature values.
The discrepancy in the case of glass might be dirt on the glass
surface. |
 |
  4
Tips and Comments 4
Tips and Comments
|
 |
- Use a camera to
calculate the contact angle accurately. The contact angle
can be calculated fairly accurately with the aid of a computer.
- Color
the water droplet with methylene blue dye to accentuate
the contours of the water droplet and to make observations
easier.
That will greatly help determining the contact angle with
the aid of the camera.
The silicone fluid can be baked into the glass and then
rubbed off with a paper towel so that the coating is almost
invisible.
Nevertheless, the water-repellent effect can still be observed.
Baking can be done by thoroughly cleaning the silicone-treated
glass and dry heating it at over 100 °C for 15 to 20
minutes.
- A qualitative version of the experiment (without a camera)
is easy and quick to perform. The experiment is thus
ideal for demonstrating
the water-repellent properties of silicones, and especially
of silicone fluids. Qualititative determination of the
contact angle
with a camera is fairly time-consuming and offers little
in the way of new insights, as the result can be interpreted
qualitatively.
- Comparing the molecular structures of silicone
fluid, glycerol and paraffin wax is a very good way of
explaining the different
properties. This affords a good way of incorporating
the important relationship between a material’s particle
structure and its properties into the lesson.
- In secondary
school chemistry classes, the experiment could be used,
for example, to cover the terms hydrophobic and
hydrophilic. It could also be used as part of a project.
Advanced chemistry students could draw on their knowledge
of particle structure and properties (water repellency
of alkanes)
to interpret the results and deepen their understanding.
In the construction industry, the water-repellent property
of
silicone
fluid is a key element of monument conservation ("Silicones
for construction")
- Since the experiment is safe and
simple, it is ideal for the classroom. To save time,
the pupils should be divided
into
groups, with each group investigating a different
surface.
|
 |
  5
Supplementary Information 5
Supplementary Information
|
 |
The organic methyl groups contained
in silicones are very hydrophobic (water repellent), because
there is extremely little interaction between the methyl groups
and the water molecules.
A measure of the hydrophobic properties of a material is its
tendency to cause wetting, i.e. the ability of liquids (in this
case, water) to form a boundary with solids.
The wetting tendency can be derived from the contact
angle θ formed between
the water and the solid surface. The higher the contact angle,
the less interaction there is between the solid and the
water. |
Contact angle  of
a water drop on a (water-repellent) surface of
a water drop on a (water-repellent) surface |
 |
|
 |
 |