 |
Rate of Hydrolysis of Chloromethylsilanes |
 |
  1
Materials, Chemicals, Time Needed 1
Materials, Chemicals, Time Needed |
 |
- Retort
- Retort clamps and sleeves
- Magnetic hot plate
- Magnetic stirrer bar
- Thermometer (measuring up to 100 °C)
- Conductivity meter
- Voltmeter (3 V ~)
- Ammeter (30 mA ~)
- Variable ac source
- Stop-watch
- 3 glass beakers, 250 ml, tall
- Glass beaker, 25 ml
- 3 test tubes
- 3 suitable rubber stoppers
- Test-tube rack
- Balance
- Small conical flask
- 3 pipets (15 ml, 20 ml, 25 ml)
- Pipet bulb
- Chlorotrimethylsilane (M), C, F
- Dichlorodimethylsilane (D), Xi, F
- Trichloromethylsilane (T), Xi, F
- Universal indicator paper
- Hydrochloric acid, c = 2 mol/l
The whole experiment takes about 35 minutes. Allow 20 minutes
for preparation, including calibrating the measuring equipment,
11 minutes for the actual hydrolyses and the rest for rearranging
and intermediate cleaning. |
 |
  2
Procedure and Observations 2
Procedure and Observations |
 |
As chloromethylsilanes
hydrolyze very easily to release hydrogen chloride, perform
the experiment in a fume cupboard.
Wear safety glasses, rubber gloves and laboratory coat.
Introduce 50 ml water into each of three glass beakers.
Measure each of the chloromethylsilanes into a test tube
and keep it ready: add 10.86 g chlorotrimethylsilane to the
first, 6.45 g dichlorodimethylsilane to the second and 4.98
g trichloromethylsilane to the third test tube. Seal the
test tubes. Set up the measuring apparatus in a fume cupboard
as shown in the following sketch. |
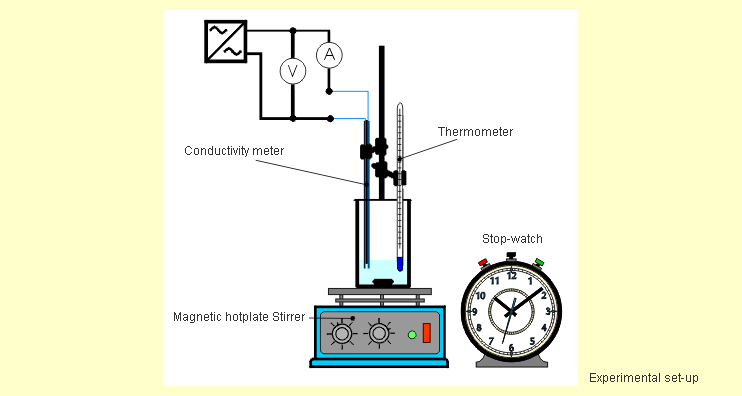 |
  Before
conducting the individual measurements, dip the conductivity
meter in hydrochloric acid, c = 2 mol/l, and adjust the voltage
until a useful current reading (from 10 mA to 15 mA) is obtained
without any visible gas formation on the electrodes. Clean the
conductivity meter and suspend it in the water for the first
hydrolysis measurement. Set the stirrer to a medium speed. Start
the stop-watch at the same time as adding the chlorotrimethylsilane
and observe the change in current strength. After the current
has remained constant for 60 seconds, stop the experiment. Do
not change the set voltage on the power supply. Before
conducting the individual measurements, dip the conductivity
meter in hydrochloric acid, c = 2 mol/l, and adjust the voltage
until a useful current reading (from 10 mA to 15 mA) is obtained
without any visible gas formation on the electrodes. Clean the
conductivity meter and suspend it in the water for the first
hydrolysis measurement. Set the stirrer to a medium speed. Start
the stop-watch at the same time as adding the chlorotrimethylsilane
and observe the change in current strength. After the current
has remained constant for 60 seconds, stop the experiment. Do
not change the set voltage on the power supply.
Clean the conductivity meter and the thermometer and set up the
experiment again for the next beaker of water. Perform the second
measurement on dichlorodimethylsilane.
In both cases, the reaction mixture turns hazy when the chloromethylsilane
is added, the current strength increases rapidly and reaches
a constant value after about 25 seconds. When the reaction mixture
is left to stand for a prolonged period, two liquid phases separate
out.
Do not use the conductivity meter when hydrolyzing trichloromethylsilane
as it might become damaged. Hydrolysis, and the subsequent condensation,
occurs as soon as the trichloromethylsilane is added. A solid
silicone is produced that would stick to the contacts of the
conductivity meter.
The reaction conditions are summarized below:
- Chlorotrimethylsilane 10.86 g + 50 g water
- Dichlorodimethylsilane
6.45 g + 50 g water
- Trichloromethylsilane 4.98g + 50 g water
(current is not measured in this case)
- Room temperature 295
K
- Measuring voltage U = 1 V
- Measured current in HCl (aq), c
= 2 mol/l: I = 10 mA
  The
experimental results are summarized in the following table: The
experimental results are summarized in the following table:
|
 |
Time in s |
Chlorotrimethylsilane
Current in mA
|
Dichlorodimethylsilane
Current in mA
|
5 |
6 |
10 |
10 |
9 |
10.5 |
15 |
10 |
10.5 |
20 |
10.5 |
10.5 |
25 |
10.5 |
10.5 |
30 |
10.5 |
10.5 |
60 |
10.5 |
10.5 |
120 |
10.5 |
10.5 |
180 |
10.5 |
10.5 |
240 |
10.5 |
10.5 |
300 |
10.5 |
10.5 |
|
 |
  3
Discussion of Results 3
Discussion of Results
|
 |
| None of the three chloromethylsilanes
is resistant to water and all three hydrolyze to methylsilanols
and hydrogen chloride. The equation for the reaction of dichlorodimethylsilane
is shown here as an example: |
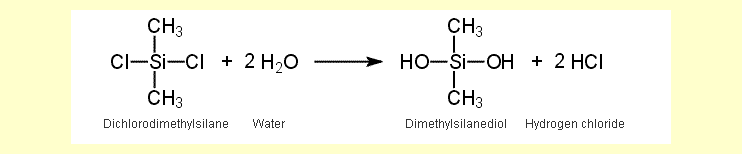 |
The chloride and hydronium ions
formed cause the current strength to increase in proportion to
their concentration. The change in current strength is therefore
a direct measure of the state of the reaction and may be used
to determine the rate of the reaction. The quantities of materials
used in the experiment are calculated to produce hydrochloric
acid in a concentration of c = 2 mol/l in each glass beaker if
hydrolysis goes to completion.
A plot of the readings yields the following current-time curves: |
 |
 |
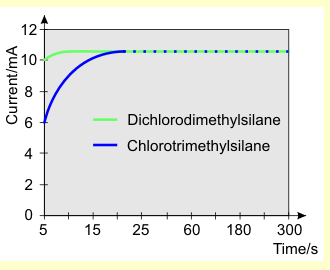 |
It is clearly evident that chlorotrimethylsilane
hydrolyzes somewhat more slowly than dichlorodimethylsilane,
but the reaction is still fairly rapid (it is complete
after 25 seconds). The amperometric measurement provides
no information about the speed at which the silanols formed
during the hydrolysis condense. A qualitative impression
is given, however, by the phase separation that occurs
after hydrolysis (see above).
Whereas the condensation of
monofunctional trimethylsilanol finishes with the formation
of low-viscosity hexamethyldisiloxane,
the other two condensation reactions yield high-viscosity
and solid products. |
|
| The equation for chain formation
during the condensation of dimethylsilanediol is: |
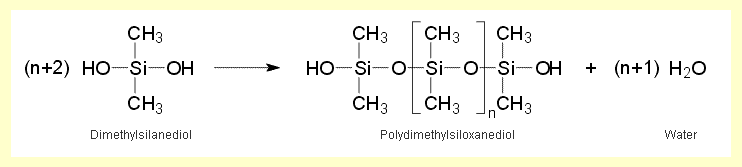 |
 |
  4
Tips and Comments 4
Tips and Comments
|
 |
- On the basis of the results,
only the hydrolysis of chlorotreimethylsilane is recommended
for amperometric measurements – the other two chloromethylsilanes
hydrolyze too quickly.
- The structural similarity of chlorotrimethylsilane
and 2-chloro-2-methylpropane (tert.-butyl chloride) is
reason enough to perform the experiments
described here on the two chlorine derivatives with a view
to comparing nucleophilic substitution at the Si atom and
at the
C atom.
- All hydrolysis experiments described here are exothermic.
If the experiment is started at room temperature, the measurements
will show a rise in temperature to about 40 °C.
Some of the dichlorodimethylsilane hydrolysis product may
be used after brief conditioning in the experiment "Burning
of liquid silicones".
- The dichlorodimethylsilane hydrolysis
products are suitable for the experiment "Viscosity
of silicone fluids", especially
if samples from previous experiments that have been standing
around for some time are available.
|
 |
  5
Supplementary Information 5
Supplementary Information
|
 |
| Given the outstanding importance
of hydrolysis and subsequent condensation of chloromethylsilanes
in the synthesis of silicones, these simple, yet informative
experiments should be taught in lectures and used for practical
work at university, as well as for advanced chemistry students
at school. In the form described here, they complement the information
on nucleophilic substitution and industrial production of silicones,
which is contained in the textbook indicated below. |
 |
  6
References 6
References |
 |
| M. Tausch, M. von Wachtendonk
(editors), CHEMIE S II, STOFF-FORMEL-UMWELT, C.C. Buchner, Bamberg
(1993), (1998), S. 337f |
 |
 |
|