 |
Siliconized Filter Paper |
 |
  1
Materials, Chemicals, Time Needed 1
Materials, Chemicals, Time Needed |
 |
- Filter funnel tripod
- 2 fast-flow filter funnels
- 2 fluted filter
papers
- 4 test tubes
- Glass beaker, 50 m
- Pasteur pipet with rubber
cap
- Copper sulfate, Xn, N
- Dichloromethane
(methylene chloride), Xn
- Trichloromethylsilane, Xi, F
Allow about 10 minutes for silanizing the
filter paper. The experiment itself takes less than 5 minutes.
|
 |
  2
Procedure and Observations 2
Procedure and Observations |
 |
Using the pasteur pipet, wet one of
the fluted filter papers with trichloromethylsilane in
a fume cupboard. Ensure that the entire filter paper is
wetted with trichloromethylsilane. Place the filter paper
aside to dry.
While it is drying, dye approx. 15 ml water
with copper sulfate. Fill two test tubes to a depth
of around two centimeters
with the colored water and add roughly the same amount
of organic solvent (dichloromethane). Two phases form
because the liquids are immiscible. Dichloromethane is
denser than
copper sulfate solution (colored water) and thus forms
the lower layer (see Fig. 1).
|
 |
 |
 |
|
Fig. 1. Test tubes containing test liquids |
|
 |
 |
Fig. 2. Filtrates from the two filters |
 |
| Now assemble the apparatus as shown in Fig.
2 and add the contents of one test tube to the “untreated
funnel” and the contents of the other to the (dry) “siliconized
funnel.” The water passes through the “untreated
funnel” as usual, and the dicholoromethane remains in the
funnel. In the case of the siliconized paper, the colored water
remains in the funnel and the dichloromethane passes through
(see Fig. 3). |
 |
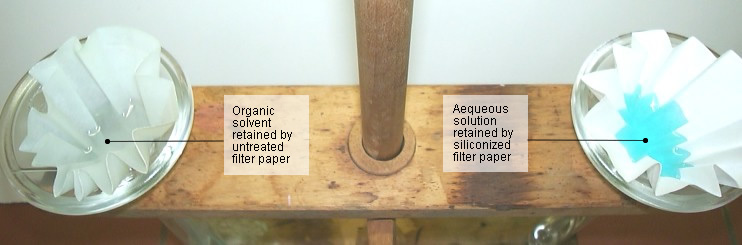 |
| Fig. 3. Residues on the two filter papers |
 |
  3
Discussion of Results 3
Discussion of Results
|
 |
| Standard laboratory filter paper
consists of cellulose, whose simplified structural formula is
shown in Fig. 4. |
 |
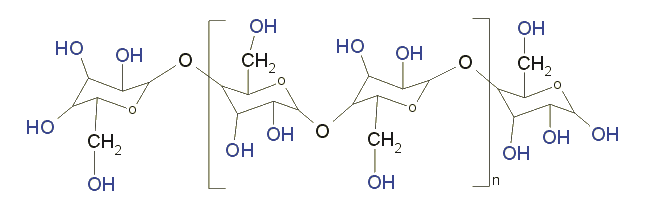 |
| Fig. 4: Schematic diagram of a cellulose
molecule |
 |
| Water consists of polar molecules.
The molecules of the cellulose contain many hydroxy groups that
can form hydrogen bonds and undergoes dipole-dipole interactions
with water molecules. Water therefore penetrates into the hydrophilic
cellulose and passes through the filter paper. The hydrophobic
dichloromethane does not penetrate into the cellulose filter
paper. When the filter paper is wetted with trichloromethylsilane,
condensation reactions occur, with a large number of the hydrogen
atoms in the hydroxy groups being replaced by methylsilyl groups.
This also causes interlinking of the cellulose molecules (see
Fig. 5 and 6). The product contains numerous nonpolar methyl
groups that make it hydrophobic and lipophilic. |
 |
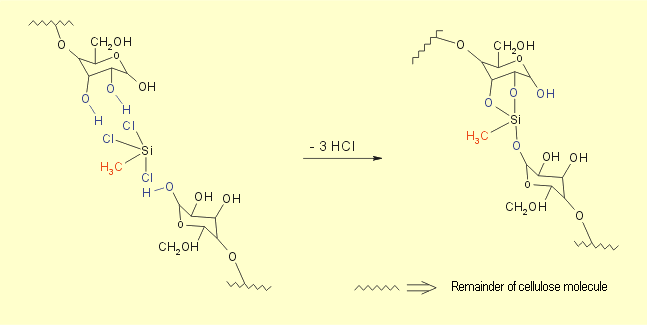 |
| Fig. 5: Condensation reaction between
cellulose and trichloromethylsilane |
 |
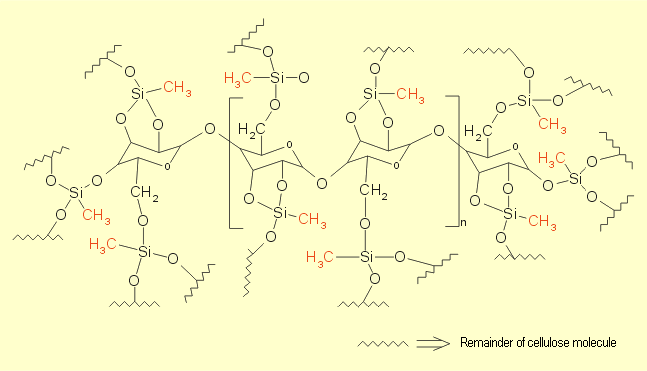 |
| Fig. 6: Schematic diagram of siliconized
cellulose |
 |
| The resultant siliconized cellulose
is no longer permeable to water. However, a nonpolar or weakly
polar solvent that cannot form hydrogen bonds, e.g. dichloromethane,
is able to flow through the siliconized cellulose. |
 |
  4
Tips and Comments 4
Tips and Comments
|
 |
- Additional minor experiments could be performed
to show that HCl is released when the filter paper is treated
with trichloromethylsilane. This may be done by washing
the paper with distilled water after siliconization and detecting
hydronium
ions in the water (with acid-base indicator) and detecting
chloride ions with silver nitrate solution.
- In the lesson, the
pupils should suggest this identification
reaction on the basis of the reaction scheme provided
in Fig. 5, and should then perform it.
The mechanism of nucleophilic substitution may be discussed
in connection with the reaction that occurs during siliconization
(Fig. 5).
- In addition to the variant described here, filter paper
could also be siliconized with a) dichlorodimethylsilane
and b) chlorotrimethylsilane.
The observations made could then be discusssed in class.
|
 |
  5
Supplementary Information 5
Supplementary Information
|
 |
Although this experiment bears a certain resemblance to the "Silicon-coated
paper", there is a fundamental difference: When the filter
paper in this experiment is siliconized, the cellulose reacts
with the added trichloromethylsilane and the molecular structure
of the paper is radically altered (see Figs. 4, 5 and 6). In
the other experiment, when the paper is coated with silicone,
the silicone has already been crosslinked and is applied in
the form of a thin film; the vast majority of the paper’s
molecular structure remains unchanged. |
 |
 |