 |
Viscosity of Silicone Fluids/Variant
B |
 |
  1
Materials, Chemicals, Time Needed 1
Materials, Chemicals, Time Needed |
 |
- Retort
- Retort clamps and sleeves
- Magnetic hotplate and stirrer bar
- Magnetic stirrer bar
- Glass beaker, as large and tall as possible
- Glass tube (50
cm x 5 mm diameter)
- Rubber stopper
- Steel ball (4 mm diameter)
- Thermometer (100 °C)
- Stop-watch
- Magnet
- Small funnel or a pipet for filling the tube
- Fine permanent
felt-tip marker
- Angle-measuring scale (triangle)
A single series of experiments can
be conducted in 15 minutes
at room temperature.
Other series of measurements
take more time because the
tube has
to be rinsed,
dried
and then equilibrated
again
each
time. Fifteen minutes should
be long enough to prepare for
the
next
series.
If a measurement series is
to be recorded at different
temperatures,
the time
required depends
chiefly
on the time needed to heat
up and cool down the water
bath.
|
 |
  2
Procedure and Observations 2
Procedure and Observations |
 |
 |
 |
 |
Set up the apparatus as shown in
the diagram. Clamp the tube at a slight angle, but ensure
that
neither it nor the stopper make contact with the glass
beaker. The precise angle is not critical. However, whatever
angle is chosen, ensure that all measurements are made
at that angle (the angle for precision measurements laid
down in standards is 10°).
Make two marks on the glass
tube, one about 3 cm beneath the water surface and
the other 1.5 cm above the stopper.
Fill the tube with the test liquid, ensuring there
are no bubbles, clamp the tube in position and add the
ball.
The entire apparatus must be equilibrated in the measurement
range for at least 5 minutes before the measurements
are started. For the measurement, use the magent, which
has
also been equilibrated, to draw the steel ball up to
about 1 cm below the water surface. |
|
| Then remove the magnet and measure the time
for the ball to fall between the two markings. Viscosity measurements
make it possible to distinguish between the different samples. |
 |
 |
 |
An interesting experiment is to
measure the viscosity change that occurs on aging (use
samples from the experiment "Rate
of hydrolysis of chloromethylsilanes"). That provides evidence
of progressive condensation. It is also interesting to
compare the viscosity changes that occur in silicones
and an organic oil as the temperature changes. The apparatus
shown is ideal for distinguishing between the silicone
fluids on the basis of their viscosity as measured in
terms of the “fall time.” It turns out that
silicone fluids with high molar masses have a greater
viscosity and that the ball needs more time to cover
the distance than it does in silicone fluids of low molar
mass. When the hydrolysis products obtained in the experiment "Rate
of hydrolysis of chloromethylsilanes" are used,
the hexamethyldisiloxane is found to have a very low
viscosity, whereas the dichlorodimethylsilane hydrolysis
products generally have a higher and, more importantly,
increasing viscosity as they age.
The viscosity of all
samples decreases with rising temperature. However,
the viscosity of olive oil is much more dependent
on the temperature than that of silicone fluids. In the
case of olive oil, the fall time is reduced by 80 % as
the temperature is increased to 65 °C compared with
a reduction of only 50 % for silicone fluids over the same
temperature interval (see below).
|
|
 |
  3
Discussion of Results 3
Discussion of Results
|
 |
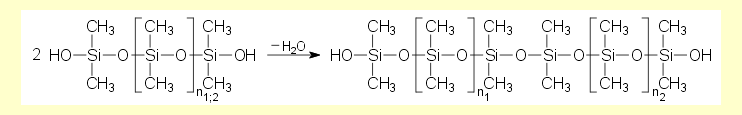
| The experimental fall times for
the ball correlate with the viscosity values of the fluids studied
(the longer the fall time, the higher is the viscosity of the
fluid). The higher the molar mass of the silicone fluids (i.e.
the larger or longer the silicone molecules), the greater is
their viscosity. This is shown, for example, by the hydrolysis
product of dichlorodimethylsilane, whose viscosity increases
with increasing age, i.e. with the degree of condensation. |
 |
 |
 |
| The comparatively low
temperature dependence of the viscosity of a silicone fluid,
relative to that of olive oil, is clearly revealed by this
experiment. Whereas the fall time for the ball is shortened
by 80 % in olive oil, the corresponding figure for silicone
fluid over the same temperature interval is just 50 % (see
diagram). |
 |
|
 |
  4
Tips and Comments 4
Tips and Comments
|
 |
| See Tips
and comments under Variant A. |
 |
  5
Supplementary Information 5
Supplementary Information
|
 |
| See Supplementary
information under Variant A. |
 |
 |