 |
Viscosity of Silicone Fluids/Variant
A |
 |
  1
Materials, Chemicals, Time Needed 1
Materials, Chemicals, Time Needed |
 |
- Laboratory stand
- Retort
- Magnetic hot plate and stirrer bar
- Glass
beaker, 1 l
- Glass tube (length = approx. 50 cm, Ø =
6 mm)
- 2 metal balls ( Ø = 3.5 mm and 5.5
mm)
- Thermometer (100 °C)
- Stop-watch
- Magnet
- Small plastic funnel
- Fine permanent felt-tip
marker
- Triangle
- Rubber stopper
A single measurement at
a given temperature takes about 5 minutes. The total time
needed for the measurement series, which involves
different temperatures, depends
essentially on the heating and cooling rate of the
waterbath and is hard to
predict.
In general, given a methodical
approach,
1.5 hours should suffice
for the series of measurements
(see 4 Tips and
comments).
Series of measurements on other oils
take more time because the tube has
to be rinsed,
dried and then
equilibrated again each time.
|
 |
  2
Procedure and Observations 2
Procedure and Observations |
 |
 |
 |
Set up the apparatus as shown in
the diagram.
Clamp the glass tube at a slight angle of around 10°.
The precise angle is not critical for the calculation later
because it is allowed for in the apparatus constant. However,
to ensure that the readings are comparable, all measurements
must be carried out at the exact same angle. This is achieved
by keeping the retaining clamp in one position. Do not
change its position throughout the experiment.
Before the
glass tube is clamped in position, make two marks on
it, one about 3 cm beneath the water surface and
the other 1.5 cm above the rubber stopper.
Then fill the
tube with the respective oil, ensuring there are no
bubbles, add the steel ball and clamp the
tube in
position.
The glass tube must be equilibrated at this temperature
for at least 5 minutes before the measurements are
started.
|
 |
|
| For the measurement itself, use the magnet,
which has also been equilibrated, to draw the steel ball up to
about 1 cm below the water surface and then measure the time
for the ball to fall between the two markings. Take five readings
at each temperature. |
 |
 |
 |
 |
|
If the “fall time” for
the larger ball is too long, use the smaller one. Doing
this does not affect the temperature dependence.
For each oil (see list of chemicals), determine the fall
time at five temperatures roughly 10 °C apart (approx.
10 °C, room temperature, 35 °C, 45 °C, 55 °C).
It will be seen that the fall time decreases in all oils
as the temperature rises.
The fall time in silicone fluids is much longer than in
the other oils.
|
|
 |
  The
following fall times were measured in a reference experiment: The
following fall times were measured in a reference experiment: |
 |
|
 |
| As may be seen, there is very good reproducibility
among the readings. |
 |
  3
Discussion of Results 3
Discussion of Results
|
 |
| The natural logarithm of the
fall time decreases linearly with rise in temperature. This is
illustrated in the diagram with the readings from the reference
experiment. |
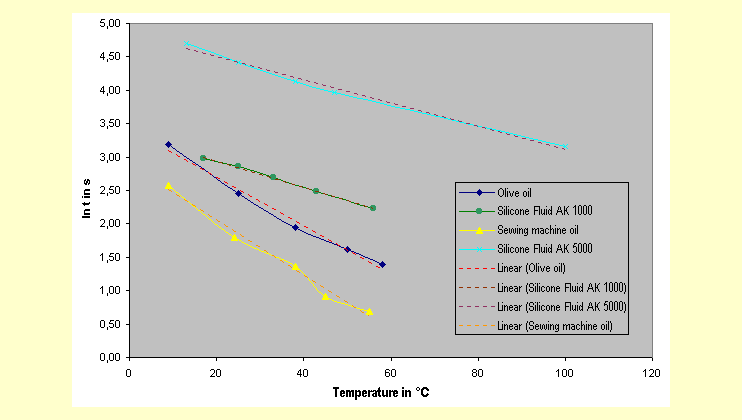 |
Additionally, it may be seen
from the slope of the curves that the viscosity (which is proportional
to the fall time measured here) is not so dependent on the temperature
in the case of the silicone fluids as it is with the other oils.
This property of silicone fluids is a key factor in their use
in hydraulic equipment that must function reliably at temperatures
that vary considerably. One such case is airplanes, where mineral
oils would be unsuitable because their viscosity is too dependent
on the temperature. |
 |
  4
Tips and Comments 4
Tips and Comments
|
 |
- For school use, it is advisable
to dispense with calculating the viscosity from the fall
time readings and simply to convey that viscosity and fall
time
are related to each other in the form of “the higher,
the longer.”
- It
is best to leave the fall times in silicone fluid until
last as silicone fluid is hard to remove from the glass tube.
To shorten the experiment time, it is advisable to keep
two very large glass beakers containing very hot and
cold water
handy
so that the temperatures for making the readings can
be adjusted quickly.
- Metal balls of different sizes can be obtained in
bicycle shops, for example.
- The experiment illustrates clearly
that the viscosity (fall time) of silicone fluids is
less dependent on the temperature
than
it is for other oils. This may be explained in terms
of their linear structure and the comparative lack of interactions
between silicone molecules.
- The experiment is suitable for
introducing advanced chemistry students to the concept
of viscosity. Normally, this is
only done with organic oils. Silicone fluid makes
a good oil for
comparison because of its special properties and
its importance in industry.
- The experiment is ideal for classrooms
because it is easy to conduct and the chemicals employed
are relatively
safe.
To save
time, groups of pupils could study a different
oil.
- To enhance the pupils’ computer skills, a computer
should be used for the evaluation. The evaluation could also
be set as homework.
|
 |
  5
Supplementary Information 5
Supplementary Information
|
 |
Viscosity (internal friction)
is the property of a liquid to resist mutual displacement of
two adjacent layers. Nowadays, the so-called dynamic viscosity η =
t/D is defined as the ratio of the shear stress to the
velocity gradient parallel to the direction of flow.
In Newtonian fluids, the viscosity at a given temperature is a property
of the material. That is not true of non-Newtonian fluids. With non-Newtonian
fluids, the flow rate governs whether the viscosity decreases or increases.
These phenomena are observed, for example, when liquid pastes (e.g. tooth
paste, artists’ colors) are squeezed from tubes and spread quickly. |
 |
  6
References 6
References |
 |
| A. Tomanek: Silicones & Industry,
A compendium for practical use, instruction and reference, Hanser,
Münich, Vienna (1990) |
 |
 |