 |
Hydrolysis of Triacetoxymethylsilane |
 |
  1
Materials, Chemicals, Time Needed 1
Materials, Chemicals, Time Needed |
 |
- Watchglass
- Dropping pipet
The experiment takes less than five minutes.
|
 |
  2
Procedure and Observations 2
Procedure and Observations |
 |
 |
 |
 |
In a fume cupboard, add about 20 drops of water to about
10 drops of triacetoxymethylsilane on a watchglass.
Observe
the white “strings” in the liquid
that form a few seconds after the water has been added.
When the liquid has evaporated, a thin hazy layer remains
firmly stuck to the watchglass and is highly water repellent.
|
|
 |
  3
Discussion of Results 3
Discussion of Results
|
 |
The triacetoxymethylsilane hydrolyzes
in water to form a methylsilicone resin and acetic acid.
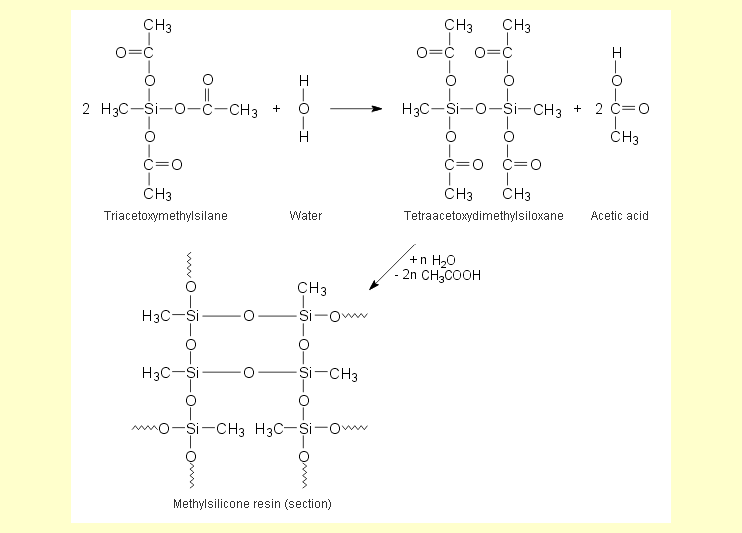
If the product from the experiment “Synthesis
of triacetoxymethylsilane” is
used, the acetic anhydride still contained in it must be completely
converted to acetic acid. That is why the water is added in
excess.
The silicone resin produced by the hydrolysis adheres well
to silicate glass and is hydrophobic (see also the experiment "Hydrophobic
properties of silicone fluid"). |
 |
  4
Tips and Comments 4
Tips and Comments
|
 |
- This experiment is of relevance to everyday life. It may
be performed on silicone bought from a DIY (Do it yourself)
store instead of the
triacetoxymethylsilane that you have produced (see 5
Supplementary Information).
- Acetoxysilanes are much more easily hydrolyzed
than esters, such as ethyl acetate. They hydrolyze as easily
as acetic anhydride.
This could be developed into a topic that also deals with
the mechanism of ester hydrolysis (see also 6
References).
|
 |
  5
Supplementary Information 5
Supplementary Information
|
 |
| The typical vinegar odor smelled
when silicone sealants from DIY stores are used stems from the
reaction above. The commercial tubes contain silicone material
whose macromolecules have a fairly high content of acetoxy groups.
When the sealant is squeezed from the tube into the air, the
macromolecules undergo hydrolytic crosslinking in the atmospheric
moisture, and acetic acid is released. |
 |
  6
References 6
References |
 |
| M. Tausch, M. von Wachtendonk
(editors), CHEMIE S II, STOFF-FORMEL-UMWELT, C.C. Buchner, Bamberg
(1993), (1998), S. 267 - 269 |
 |
 |