1. Experiment is contained in the WACKER's Experimental Kit. |
No |
2. Experimental procedure has been modified |
/ |
3. A separate experimental procedure has been devised |
Yes |
4. Video clip available |
No |
5. Flash animation available |
No |
 |
Synthesis of Triacetoxymethylsilane |
 |
  1
Materials, Chemicals, Time Needed 1
Materials, Chemicals, Time Needed |
 |
- Retort
- Retort clamps and sleeves
- Laboratory stand
- Magnetic hot plate
- Small magnetic stirrer bar
- Oil bath
- Round-bottom flask (50 ml)
- Stoppers
- Reflux condenser with tubing
- Thermometer (200 °C)
- 3 graduated measuring pipets (5 ml, 1 ml, 1 ml)
- Pipet bulb
- Angle adapter for the top of the condenser
- Glass rod
- Acetic acid, C
- Acetic anhydride, C
- Trichloromethylsilane, Xi, F
- Silver nitrate solution, Xi
The whole experiment takes about 40 minutes. Allow 20 minutes
for setting up the apparatus
and heating the oil bath, 15 minutes for the reaction,
and 5 minutes
for
cooling
and decanting
the
product.
|
 |
  2
Procedure and Observations 2
Procedure and Observations |
 |
 |
 |
As highly corrosive chemicals are used, some of which
are gases, perform the experiment in a fume cupboard.
Wear safety glasses, rubber gloves and a laboratory coat.
Set up the apparatus in the fume cupboard as shown
in the adjacent diagram. Then mix 2.5 ml acetic acid
with 0.83 ml acetic anhydride and 0.8 ml trichloromethylsilane
and heat under reflux with stirring at 120 °C to
130 °C. Store the cooled product away from moisture
until needed. It is suitable for the experiment "Hydrolysis
of triacetoxymethyl silane".
|
 |
|
  3
Discussion of Results 3
Discussion of Results
|
 |
Under the action of heat, the
chlorine atoms on the trichloromethylsilane are replaced by acetoxy
groups.

Hydrogen chloride is released and escapes as a gas. The mixture of the
principal product triacetoxymethylsilane, the reagents and the byproducts
remains as a liquid in the apparatus. Acetic anhydride reacts with the
atmospheric moisture entering the apparatus and the water already present
in the acetic acid to form acetic acid and thereby prevents premature hydrolysis
of the triacetoxymethyl silane (see also the experiment "Hydrolysis
of triacetoxymethyl silane").
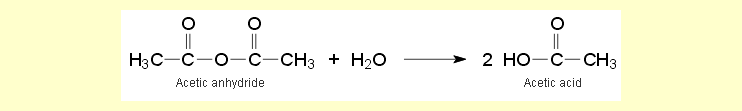 |
 |
  4
Tips and Comments 4
Tips and Comments
|
 |
- With a bit of dexterity, chloride
ions may be detected at the top of the reflux condenser
with a drop of silver nitrate solution on the glass rod. Place
a manifold
on the condenser before doing this test because it is essential
to prevent any silver nitrate solution from falling into
the reaction solution.
- The principal reaction, between trichloromethyl
silane and acetic acid (see above), may be interpreted
and explained as a condensation
reaction as well as a nucleophilic substitution reaction.
- Continuous
removal of one product (the hydrogen chloride) from the
reaction mixture continually upsets the equilibrium
shown
above; this is one way of quantitatively converting
at least one of the reagents, even though an equilibrium would
be
established in a closed system.
- This experiment may be combined
with the experiment to hydrolyze the triacetoxymethylsilane
you have produced
with a view
to discussing in more detail the italicized terms
above and to illustrating
products of practical relevance. More details in
this regard may be found in the textbook mentioned below (via
the
key
words mentioned).
|
 |
  5
Literatur 5
Literatur |
 |
| M. Tausch, M. von Wachtendonk
(editors), CHEMIE S II, STOFF-FORMEL-UMWELT, C.C. Buchner, Bamberg
(1993), (1998) |
 |
 |
|