  Chloromethylsilanes:
Silicone Precursors with Interesting Properties Chloromethylsilanes:
Silicone Precursors with Interesting Properties
The
mixture of products generated by the Müller-Rochow synthesis contains a number of different chloromethylsilanes that are
important to the chemical industry. These are:
- Chlorodimethylsilane ((CH3)2HSiCl;
Bp: 35 °C)
- Chlorotrimethylsilane ((CH3)3SiCl;
Bp: 57 °C)
- Dichloromethylsilane ((CH3)HSiCl2;
Bp: 41 °C)
- Dichlorodimethylsilane ((CH3)2SiCl2;
Bp: 70 °C)
- Trichloromethylsilane ((CH3)SiCl3;
Bp: 66 °C).
The number of chlorine atoms can be used
to distinguish the functionality of the monomer units above.
When the chloromethylsilanes are crosslinked, the individual
chlorine atoms are replaced by hydroxyl groups first, with
release of hydrogen chloride. The hydroxyl groups then react
further, undergoing polycondensation to the corresponding polysiloxanes.
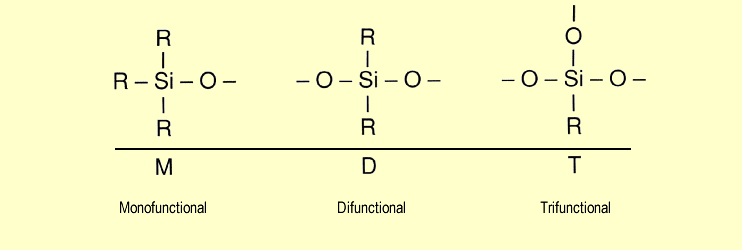
  The
following diagram shows the different functionalities of the
chloromethylsilanes in terms of the polymer units generated
from them: The
following diagram shows the different functionalities of the
chloromethylsilanes in terms of the polymer units generated
from them:
|
| It can be seen that
monochlorosilanes are monofunctional units, while dichlorosilanes
are difunctional. Similarly, trichlorosilanes form trifunctional
units. (R represents either a methyl group or a hydrogen
atom.) |
Monochlorosilanes always function as chain
terminators. Dichlorosilanes form the backbone of linear and
cyclic silicones. Trichlorosilanes enable three-dimensional
silicone networks to be synthesized.
  Chloromethylsilanes
are so important because they are highly reactive. As already
mentioned, they react violently with water, releasing hydrogen
chloride in the process. The resultant silanols have a tendency
to rapidly undergo polycondensation. The composition of the
reaction mixture determines whether the ensuing products are
silicone fluids or rubbers. Chloromethylsilanes
are so important because they are highly reactive. As already
mentioned, they react violently with water, releasing hydrogen
chloride in the process. The resultant silanols have a tendency
to rapidly undergo polycondensation. The composition of the
reaction mixture determines whether the ensuing products are
silicone fluids or rubbers.
Since hydrogen chloride (e.g. 350 liters
per kilogram of dichlorodimethylsilane) is released in the manufacture of silicones,
these processes have to be carried out with extreme caution.
To accommodate environmental concerns
and process economics, the hydrogen chloride is continuously
recycled in a loop.
It is converted with methanol to form chloromethane, which
is the starting material for the Müller-Rochow
synthesis.
This example shows how the chemical industry
practices process-integrated environmental protection and operates
efficiently at the same time. The chlorine compounds remain
in the production plant during the production of silicones.
The silicones are free of chlorine. However, chlorine compounds
are still indispensable intermediates because there is still
no competitive alternative manufacturing technology for silicones.
|