  From
Raw Material to Silicones From
Raw Material to Silicones
The Element Silicon
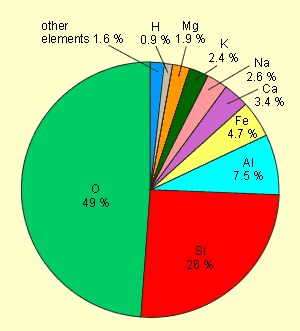
The following pie diagram shows the frequency of the elements in
the earth's crust:
Silicon makes up 26 % of the earth’s
crust where it is the second most abundant element. Although
it is much more abundant than carbon (which makes up 0.087
% of the earth’s crust), it does not play any major role
in the animate world of biomolecules. It could be said, however,
that silicon is the base material of the inanimate world. It
almost exclusively occurs in combination with the most abundant
element (oxygen) in the form of silica and silicates. Aside
from the various silicates (salts of silicic acid) with magnesium,
calcium or iron fractions, silica makes up a sizable fraction
of the earth's crust in its sand, quartz and pebble variants.
While sands and clay minerals have been processed into cultural
objects since time immemorial, it was not until 1824 that elemental
silicon in amorphous form was isolated by Berzelius. All the
silicon compounds mentioned so far have a tetrahedral structural
unit consisting of a silicon bonded to four oxygen atoms. |
 |
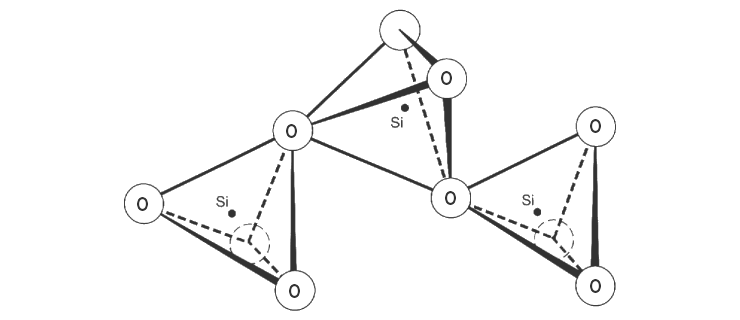 |
Structure of silicon dioxide (quartz) |
 |
Although silicon, like carbon, is in the fourth
main group of the periodic table, the two elements have vastly different
chemical properties. Carbon forms a nearly inexhaustible range of
compounds but there is no analogous range of silicon compounds. This
applies both to natural and synthetic compounds. The differences
between the two elements (carbon and silicon) are briefly illustrated
below.
  Comparison
of the Elements Carbon and Silicon: Comparison
of the Elements Carbon and Silicon:
1. Electronegativity
Carbon has a higher electronegativity than silicon (C: 2.5; Si:
1.8).
2. Atomic radii
C: 0.77 Å; Si: 1.15 Å
3. Electronic configuration
C: 1s2 2s2 2p2; Si:
1s2 2s2 2p6 3s2 3p2
4. Bonds Formed with Oxygen
Carbon tends to form double bonds with oxygen,
whereas silicon forms very stable single bonds. Silicon only forms
double bonds in the case of some unstable silane compounds. This
behavior can be understood with the help of the following molecular
orbital diagram. It is reflected in the lower energy required for  transitions
of alkenes and disilanes: transitions
of alkenes and disilanes: 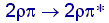 for
C, approx. 6 eV, for
C, approx. 6 eV, 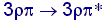 for
Si, approx. 3 eV . for
Si, approx. 3 eV .
Aside from its tetravalency, silicon is also capable of forming
compounds with higher or lower coordination numbers:
  Rough
Diagram of Industrial Silicon Synthesis Rough
Diagram of Industrial Silicon Synthesis
The other silanes are converted with alcohol to
yield resins, masonry protection agents or specialty silicones. The
interesting point about this approach is that the hydrogen chloride
gas is recycled in a continuous loop. This not only increases cost-effectiveness
but also conserves resources and considerably eases the burden on
the environment. |