| The First Step toward Silicones – Silicon from Sand
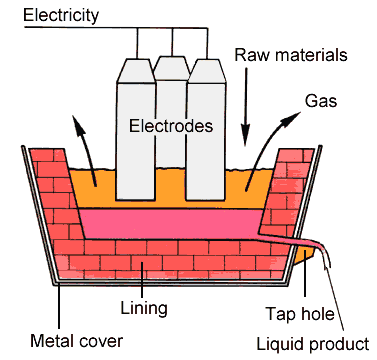
Silicon can be made in the laboratory by igniting a mixture of sand and magnesium powder with an ignition pellet: SiO2 (s) + 2 Mg (s) ® Si (s) +2 MgO (s) DH°R = -342 kJ/mol In industry, a cheaper reducing agent – carbon (coke) – is used to convert the sand into silicon. This so-called electrothermal process is carried out in gigantic furnaces in which the following endothermic reaction occurs:SiO2 (s) + 2 C (s) ® Si (s) + 2 CO (g) DH°R = + 695 kJ/mol
|
|||||||||||||
| | Home | Wuppertal University | WACKER | Didactic Dept. | Info | Experiments | Media | Work Group | Feedback | Order CD-ROM | |