  Silicones
for Paints Silicones
for Paints
Modern paints are expected to offer a range
of effects from hammer-effects to special textures. Paints
have to adhere well to different substrates. Printing inks
and paints should not foam more than necessary during manufacture
and application. And it is desirable for waterborne dyes to
be used wherever possible.
Silicone additives can meet all these requirements. Automotive
finishes illustrate this well. An automotive finish has to
meet esthetic requirements in addition to protecting the bodywork.
Colorfastness and lasting gloss are key quality determinants
here. The difference between good gloss and poor gloss is shown
in the following diagram:
Irregularities in the surface structure
of paint during finishing have various causes. These include
foaming as the paint is being sprayed and uneven evaporation
of the solvent during the drying process.
  Foaming Foaming
The finishes are sprayed in fine jets under
high pressure by automatic painting equipment. Turbulence of
the micro-droplets can cause air bubbles to be trapped, and
this can lead to flaws in the finish. With increased use of
solventless, waterborne surface-coating systems, foaming also
occurs. It is caused by the surfactants contained in the waterborne
solvents. The counter-measure is to admix silicone-based foam
inhibitors. These lower the surface tension so much that the
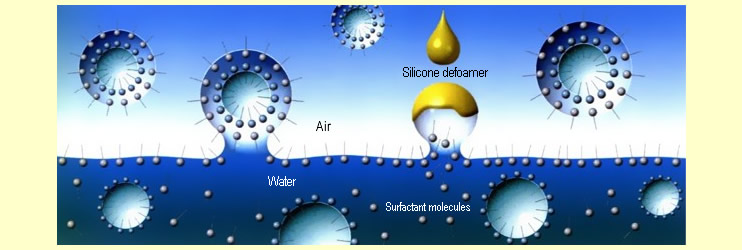
foam literally collapses. The following diagram time shows
how a silicone foam-control agent works (see also the experiment “Silicones
for antifoams”):
|
Bubbles are formed
when surfactant molecules accumulate at the boundary surface
to air, lowering the surface tension of the water and trapping
air. Provided that the surfactant doesn't drain away, the
bubbles will remain stable for quite a long time. Since
the silicone antifoams have a lower surface tension than
even the surfactants, they take their place. The foam lamella
becomes thinner as a result, becomes unstable and then
collapses.
Silicone antifoams are used wherever excessive
foaming is unwanted. Even in modern detergents.
|
  Solvent
Additives Solvent
Additives
As already mentioned, uneven evaporation
of the solvent causes irregularities in the finish. Differences
in surface tension lead to cratering by dust particles, poor
wetting of the substrate, poor mar resistance as well as flow
problems. These effects are combated by admixing silicone additives
to lower the surface tension of the paint and increase its
spreading properties. The microscopic ripple structure of the
paint becomes smoothed, incident light is reflected better
and the desired gloss effect is obtained. This shown in the
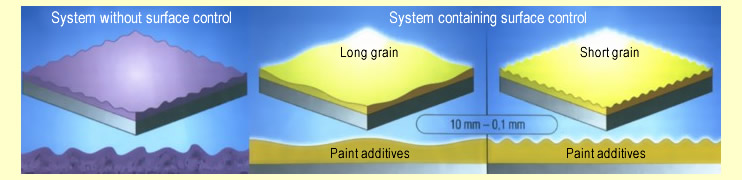
following diagram:
|
Without appropriate additives,
the dried paint surface is irregular and rough (left
diagram).
The
appropriate additives ensure that the surface appears
uniform and smooth. It is possible to create “long-wave” and “short-wave” ripple
structures by varying the type and quantity of additive
(right diagrams). |
  Heat-Resistant
Paints Heat-Resistant
Paints
Silicone-based paints have enough durability and fastness
to meet extreme demands. They are ideal for objects that have
to withstand high temperatures. These include barbecues and
all kinds of stoves as well as colored light bulbs and space
rockets.
The paint must be non-flammable, non-oxidizable and offer adequate
adhesion across all temperature ranges. Silicone
resins meet
all these requirements. A broad range of colors can be produced
by adding heat-resistant inorganic pigments . See also the
videos on this (wmv or mov).
Three Typical Applications of Heat-Resistant
Paints Are Shown Below: |
|
|