  Silicone
Resins – Hard, Insulating, Water-Repellent Silicone
Resins – Hard, Insulating, Water-Repellent
Silicone resins typically have trifunctional
and tetrafunctional units. They are basically divided into
the high-molecular forms (molecular weight: 2000 Dalton to
4000 Dalton), which are sold dissolved in organic solvents,
and the solventless liquids or powders.
Three characteristic
structures of high-molecular silicone resins are shown below:
Silicone resins are chiefly irregular networks
consisting of trifunctional structural units, as shown above.
The presence of a large number of difunctional units would
make the resins more elastic and they would then resemble silicone
rubber.
  So-called
polysesquisiloxanes have a regular structure (analogous to
that of quartz). Similar structures may be obtained by starting
from low-molecular trifunctional siloxanes (molecular weights:
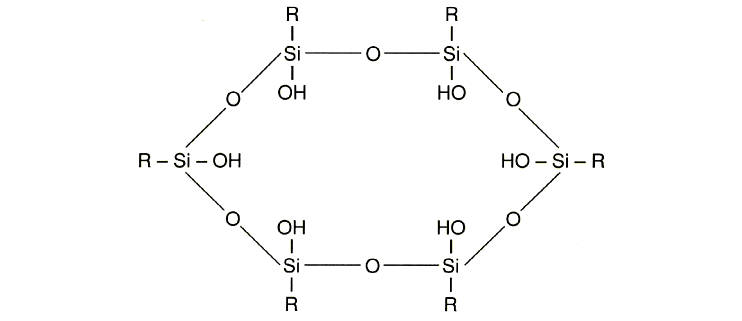
500 Dalton – 2000 Dalton). Their structure is as follows: So-called
polysesquisiloxanes have a regular structure (analogous to
that of quartz). Similar structures may be obtained by starting
from low-molecular trifunctional siloxanes (molecular weights:
500 Dalton – 2000 Dalton). Their structure is as follows:
Silicic acid esters only have a tetrafunctional
structure because they are transformed into silicic acid during
curing. Tetrafunctional units are commonly used in adhesive
silicone resins.
Ultimately, silicone resins derive their toughness from the
high degree of crosslinking undergone by trifunctional and
tetrafunctional structural units. They owe their water-repellent
properties to the presence of organic substituents. |