 |
Silicones as Antifoams |
 |
  1
Materials, Chemicals, Time Needed 1
Materials, Chemicals, Time Needed |
 |
- 2 vials with snap-on lids
- 3 test tubes and test-tube holders
- Glass tub
- Paper clip
- Dropping pipet
Allow a
total of about one hour for the following experiments. |
 |
  2
Procedure and Observations 2
Procedure and Observations |
 |
Variant A:
Take two small vials with snap-on lids, and fill each two-thirds
with water. Add several drops of detergent. Close the lids
and shake the vials vigorously. Observe how the foam changes
as time passes.
Clear solutions are formed when detergents
are added. When the vials are shaken, the upper third
fills with foam. This
initially
consists of small spherical bubbles that gradually unite
to form larger polyhedral bubbles.
Leave one of the vials containing
foam to serve as a reference.
To the other, add one drop of Antifoam Emulsion SILFOAM®SRE
from WACKER's Experimental Kit. Observe the foam.
The foam disappears very
quickly when the antifoam emulsion is added. The foam can
be heard popping as the air escapes. A slightly
hazy solution is formed. Shaking the vial again causes some
foam to form, but it collapses immediately. |
 |
  Variant
B: Variant
B:
Fill a medium-sized glass dish with water and carefully
place a paper clip (or sewing needle) on the surface of the
water. Then add several drops of water from a pipet at the
edge of the
glass dish and observe the paper clip/needle. When water is
added, the paper clip/needle continues to float
on the water.
Now add several drops of the surfactant solution
from Variant A. When the surfactant solution is added, the
paper clip/needle
slowly sinks further and further into the surface and then
suddenly sinks to the bottom. Perform the same experiment
on the second solution from Variant A (surfactant solution
+ antifoam emulsion). Again,
the paper
clip/needle sinks. |
 |
  Variant
C: Variant
C:
Thoroughly shake up a mixture of 2 ml water and 0.5
ml olive oil in a test tube. Place the test tube in the stand
and observe
how the liquids separate. Repeat the experiment on two further
samples, by adding some surfactant solution from Variant A
to one and some surfactant
and antifoam solution from Variant A to the other.
Yellowish
emulsions are formed on shaking. Without surfactant solution,
the emulsion separates again quickly and an oil phase
and a water phase are formed (see left test tube in photo
above right) With surfactant solution, the emulsion lasts longer
and
no obvious separation occurs (middle test tube). The same
observation is made for the solution containing antifoam agent.
Unlike the
pure surfactant solution, however, virtually no foam is formed
(right test tube). |
 |
  3
Discussion of Results 3
Discussion of Results
|
 |
Note: The theory behind the mechanism of surfactants and foam
formation is discussed in Part 5 Supplementary Information.
This section should perhaps be read before the discussion of Variants
A to C.
Variant A:
Surfactant solutions tend to foam.
As observed in the experiment, the bubbles are first spherical
and then polyhedral. Adding the antifoam emulsion destroys the
foam and prevents further foaming. The low amount of antifoam
added has no effect on the other properties of the surfactant
solution, such as the stabilization of emulsions
and the lowering of the surface tension of the water (see also
Variants B and C).
|
|
  Variant C: Variant C:
Surfactants can stabilize oil-in-water emulsions because their structure
allows the formation of grease/surfactant micells. That is why these emulsions
are much more durable that the pure oil-in-water emulsion. Thanks to this
effect (formation of grease/surfactant micells) and the lowering of the
surface tension of the water, surfactants constitute the most important
basic ingredient for detergents.
The surfactant solution containing antifoam
agent behaves exactly like the surfactant solution, except for the
slight foam formation. This is
due to the very good spreading power of the silicone antifoam, which
forces the surfactant particles out of the bubble skin and
thereby causes them
to collapse. |
| |
 |
  4
Tips and Comments 4
Tips and Comments
|
 |
- For the paper clip/needle experiment, ensure that they
are totally dry and clean, as otherwise they will sink immediately.
- The
experiments described here may be supplemented by those
from the booklet accompanying WACKER's Experimental Kit and from
the
CHEMIE S II textbook (see 6 References) on the subject
of surfactants.
- The antifoam experiments described here supplement
and deepen standard classroom surfactant experiments and
address the
practical requirements imposed on modern detergents.
- The
explanation of the mechanism underlying antifoams is a
prime example of the relationship between particle structure
and material properties and should also be taught as
such.
- The three variants lend themselves to classroom experiments
and could, for example, be conducted and evaluated
by the group learning method. The general theory could then
be
discussed by everyone at the end.
|
 |
  5
Supplementary Information 5
Supplementary Information
|
 |
These experiments serve to study
the influence of surfactants on the surface tension of water
and the resultant effects such as foam formation, emulsifiability
etc. In this connection, the action of silicone fluids as antifoams
is studied for its practical application.
Thus, silicone fluids are administered to cows suffering from
bloat. Bloat is a swelling of the stomach that is caused by excessive
foaming. The cause of the excessive foaming is plants that contain
sapoic acid. These react with water and intestinal gas to produce
a foam.
Silicones are especially useful here because, with a few exceptions,
they have no toxic properties at all.
The structure and mechanism of surfactants
A force is generated at the water/air interface that is based
on forces of attraction (dipole-dipole interactions and hydrogen
bonds) between the water molecules. Since each water molecule
at the surface interacts more strongly with the other water
molecules below it, i.e. inside the liquid, and beside it
in the boundary layer than it does with the molecules above
it in the air, this leads to what is known as surface tension.
It shows up as the attempt by the liquid to minimize its
surface area.
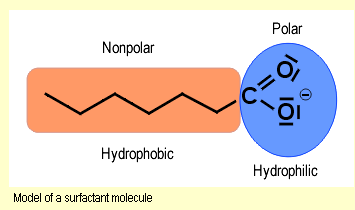
Surfactants are substances that lower the surface tension of
a liquid. As shown in the model of the surfactant particle,
it has a hydrophilic and a hydrophobic part. |
 |
 |
  At
the water’s surface, the surfactant particles align
themselves such that the hydrophilic head projects into
the water and the hydrophobic tail projects out of the
surface of the water. Since the forces of attraction between
the nonpolar hydrocarbon groups of the surfactant particles
are much lower than between the water molecules, the surface
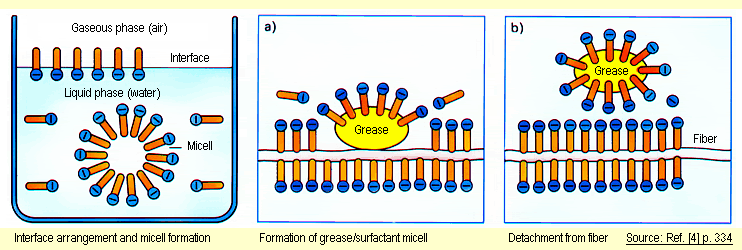
tension of the water is lowered. Inside the liquid, micells
form (see diagram below left).Emulsions are stabilized
by surfactants, dirt particles from grease and oil are
removed from surfaces and emulsified or dispersed in the
liquid phase (see diagrams a and b). At
the water’s surface, the surfactant particles align
themselves such that the hydrophilic head projects into
the water and the hydrophobic tail projects out of the
surface of the water. Since the forces of attraction between
the nonpolar hydrocarbon groups of the surfactant particles
are much lower than between the water molecules, the surface
tension of the water is lowered. Inside the liquid, micells
form (see diagram below left).Emulsions are stabilized
by surfactants, dirt particles from grease and oil are
removed from surfaces and emulsified or dispersed in the
liquid phase (see diagrams a and b). |
 |
|
 |
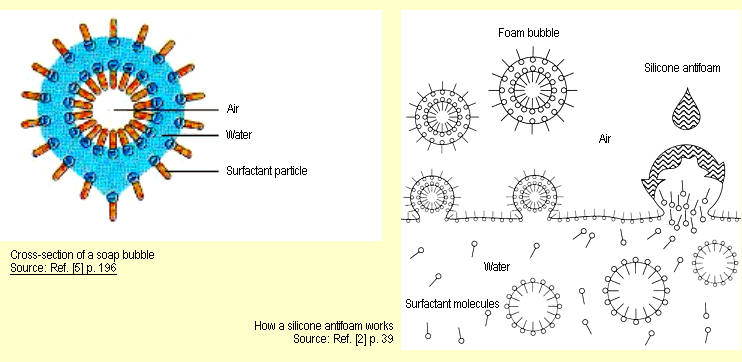
  Foam Foam
The accumulation of surfactant particles at the surface
of the water means that it becomes more and more like air, a
fact
which leads to the formation of foam bubbles (spherical foam – see
diagram below left). Progressive drainage of the liquid causes
the bubbles to become thinner and thinner. Consequently,
they huddle closer together, causing mutual deformation and
forming polyhedra (polyhedral foam – see experimental
description, Variant A) until they eventually collapse.
The air bubbles remain stable, though, if the surfactant prevents
the liquid from draining completely from the lamella.
There are applications for surfactants in which their tendency
to foam is undesirable. For example, the cleaning action of modern
washing machines relies critically on foam suppression.
Silicone fluids act as antifoams. Antifoams have a low surface
tension, poor solubility in the medium to be defoamed and positive
penetration and spreading coefficients. The particles (molecules)
of the antifoam displace the surfactant molecules from the surface
of the lamella and replace them with a new film of lower surface
tension and lower cohesive forces (see diagram below right). |
 |
| Their action can be enhanced
by adding hydrophobic solids. In such cases, the antifoam liquid
acts as a vehicle for transporting the solid particles into the
foam lamella. There, they act as foreign bodies that both absorb
the surfactant molecules and diminish the cohesive forces. Pyrogenic
silica is an ideal solid for silicone fluids. |
 |
  6
References 6
References |
 |
- M. Tausch, M. von Wachtendonk (editors), CHEMIE S II, STOFF-FORMEL-UMWELT,
C.C. Buchner, Bamberg (1993), (1998)
- M. Tausch, M. von Wachtendonk (editors), STOFF-CHEMIE S
I, FORMEL-UMWELT, C.C. Buchner, Bamberg (1996), (1997)
- W. Held et al., Learning by Doing – School
Experiments with WACKER Products (handbook accompanying WACKER's Experimental Kit), Wacker Chemie AG, Munich, 2007
|
 |
|