 |
Flammability of Silicone Rubber Compared
With That of Other Polymers |
 |
  1
Materials, Chemicals, Time Needed 1
Materials, Chemicals, Time Needed |
 |
- Bunsen burner
- Crucible tongs
- Test tubes
- pH paper
- Various plastic specimens, e.g. polyvinyl chloride
(PVC), polyethylene (PE), polypropylene (PP), polyethylene
terephthalate
(PET) and
polystyrene (PS)
- Various silicones from WACKER's Experimental Kit HTV(s), HTV(b), HTV(w), ELASTOSIL® M
4601, ELASTOSIL® M
4400, ELASTOSIL® E
43, ELASTOSIL® N
199
Allow 5 to 7 minutes for each plastic specimen.
|
 |
  2
Procedure and Observations 2
Procedure and Observations |
 |
Where possible, all experiments should be performed
in a fume cupboard. A fume cupboard must be used when PVC is
being burned!
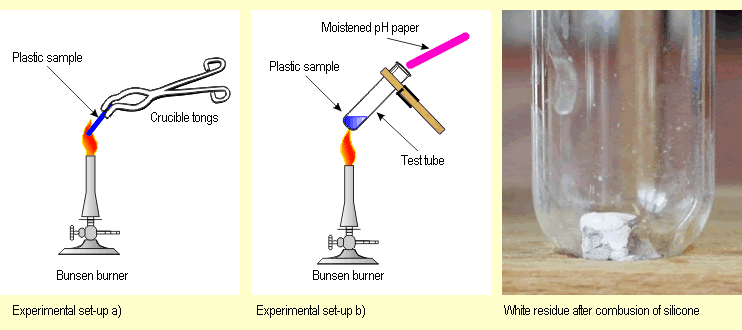
a) Using tongs, hold a piece of plastic sample for a short
time at the edge of a hot burner flame. Then observe if the
plastic continues to burn, note the color of the flame, whether
soot or fumes form, whether the flame crackles and whether
the material melts.
b) Continue by heating another part of
the sample in a test tube and carefully checking the odor.
Use damp indicator paper
to establish if the decomposition products of the reaction
are acidic, alkaline or neutral.
c) Finally, check how thermoplastic
the plastic is. Do this by using the tongs to carefully heat
another sample
of the
plastic over a small bunsen burner flame and trying to
bend the warm sample.
|
 |
  Repeat
this procedure for all the samples. Repeat
this procedure for all the samples.
The following observations
were made for the various plastic samples in several reproducible
reference experiments: |
 |
  3
Discussion of Results 3
Discussion of Results
|
 |
The various observations made
for the flame tests on the plastic samples may be explained in
terms of the elemental composition and molecular structure of
the different plastics.
As with most organic compounds, burning of organic polymers mainly
yields carbon dioxide and water, as may be seen from the following
reaction equation for the combustion of polyethylene: |
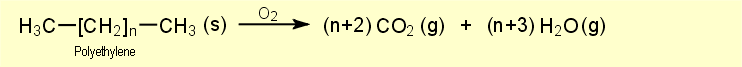 |
In addition to carbon dioxide and water, some conventional
plastics may yield other, toxic and nontoxic combustion products.
This will depend on the composition of the plastics.
An example is Polyvinylchlorid 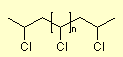 ,
which has recently hit the headlines as an environmentally polluting
plastic because it releases hydrogen chloride gas and other toxic chlorine
compounds when it burns. (Note: Special incinerators are able to burn
PVC in such a way that the environment is not polluted because the chlorine
compounds are removed from the combustion gases before they are discharged
into the atmosphere. In the experiment, the hydrogen chloride HCl formed
during the combustion of polyvinyl chloride PVC is detected with moist
pH paper, which turns red and thus indicates an acidic reaction. ,
which has recently hit the headlines as an environmentally polluting
plastic because it releases hydrogen chloride gas and other toxic chlorine
compounds when it burns. (Note: Special incinerators are able to burn
PVC in such a way that the environment is not polluted because the chlorine
compounds are removed from the combustion gases before they are discharged
into the atmosphere. In the experiment, the hydrogen chloride HCl formed
during the combustion of polyvinyl chloride PVC is detected with moist
pH paper, which turns red and thus indicates an acidic reaction.
The very
smoky flame produced when polystyrene is burned is due to the comparatively
high content of carbon (one benzene ring per monomer unit).
The following reaction scheme for the complete combustion of a silicone
shows that silicon dioxide is formed in this case as well:
|
 |
 |
 |
| Silicone fluid + oxygen |
 |
Silicon dioxide + carbon dioxide + water |
|
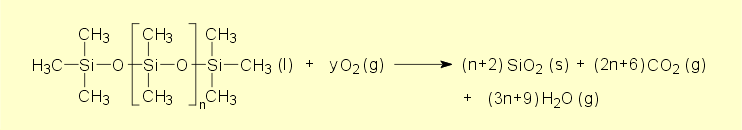 |
The observations made for the
combustion of the silicones correlate very well with the above
equation for the combustion of silicone fluid. The resultant
white product is pyrogenic silica, which is formed during the combustion.
The white smoke is also finely divided silica. Combustion or
strong heating of silicones fails to produce any basic or acidic
decomposition products.
The experiments show that silicones are much more difficult to
ignite than organic plastics, but will burn well. The activation
energy is thus very high, but combustion itself releases a great
deal of heat. This behavior is explained by the very stable Si-C
and Si-O bonds (hence the high activation energy) and the high
enthalpies of formation for SiO2 and CO2 (hence
the great deal of combustion heat). Compounds can be added to
the silicone rubber (TiO2, platinum or aluminum compounds)
to yield especially flame retardent materials which can be modified
in such a way that the flame extinguishes again after a short
time.
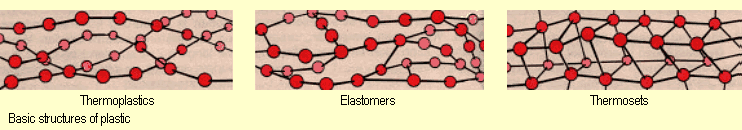
The differences in the thermoplastic behavior (see column “Flexibility” in
the table of results) may be explained in terms of the structure
of the plastics studied. All the organic polymers studied are
thermoplastics. These consist of linear or branched macromolecules
(see diagram below) that interact to a greater or lesser extent
with each other. On being heated, whole molecules or parts thereof
can slide past one another because the motion of the particles
as a whole increases and because the molecules are not crosslinked
with chemical bonds. The plastic becomes softer and pliable as
a result. When it freezes, it remains in that shape.
Unlike the organic polymers studied, the silicones examined are
elastomers or thermosets (see diagrams below). Since their macromolecules
are chemically bonded to each other, these silicones are not
thermoplastic. |
 |
 |
  4
Tips and Comments 4
Tips and Comments
|
 |
- Since there are no observed
differences among the silicones, the study can be restricted
to one type
of silicone.
- To study thermal loading, i.e. how the plastics
behave when they are heated, an alternative experimental
set-up may
be chosen
(see experiment "Burning of liquid
silicones")
- The experiment is easy to perform and
highly reproducible. It makes a good classroom experiment.
It teaches the pupils about different plastics and
how they behave during combustion. From their observations,
the pupils
come to
understand the relationship between the properties
of a
macromolecular material, its elemental composition
and its molecular structure.
- This experiment can be used as
a gateway to discuss the safety and environmental aspects
of different plastics.
|
 |
  5
References 5
References |
 |
- M. Tausch, M. von Wachtendonk (editors), CHEMIE S II, STOFF-FORMEL-UMWELT,
C.C. Buchner, Bamberg (1993), (1998), S. 337 - 352
- M. Tausch, M. von Wachtendonk (editors), STOFF-CHEMIE S
I, FORMEL-UMWELT, C.C. Buchner, Bamberg (1996), (1997), S.
228 - 233
- M. Tausch, M. von Wachtendonk (editors), CHEMIE 2000+,
C.C. Buchner, Bamberg (2001), S. 60 - 67
|
 |
 |
|