Many people have an unhealthy diet. Although we are familiar with the health risks, we eat too much “bad” fat and too little dietary fiber. For this reason, food scientists are investigating food additives which have health-promoting properties and additionally have a neutral taste and odor. The food industry uses natural cyclodextrins in a number of different ways. They meet the requirements for neutrality in terms of odor and taste since, although the cyclodextrin molecules are composed of glucose units, α- and β-cyclodextrin do not taste sweet at all, whilst γ-cyclodextrin has only a slightly sweet taste. In addition, the fact that cyclodextrins occur in the form of a colorless powder makes them easy to process. |
|||||||||||||||||||||
| α-Cyclodextrin as a Food Additive | |||||||||||||||||||||
α-Cyclodextrin is used as a prebiotic. This term is used to describe non-digestible carbohydrates which support the gut flora in the large intestine. There is considerable interest in these types of food additives, since we consume only half the amount of non-digestible carbohydrates required to maintain the health of the gut flora. The material properties of the smallest natural cyclodextrin make it ideally suited for this pharmaceutical function. Since it is readily water-soluble, does not affect the viscosity of the solution and is taste-neutral, it can be added to beverages without reservations. |
|||||||||||||||||||||
 |
|||||||||||||||||||||
| Fig. 1.44: Readily soluble α-cyclodextrin increases the fiber content of beverages without impairing the taste |
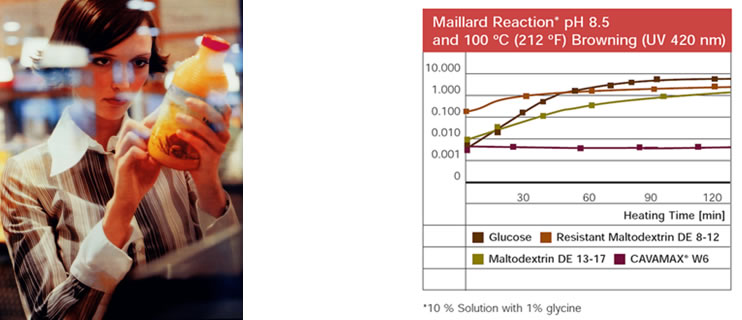
Fig. 1.45: α-Cyclodextrin produced by WACKER under the CAVAMAX® W6 brand name does not enter into the Maillard reaction with glycine | ||||||||||||||||||||
In this way we can increase the amount of dietary fiber, which is essential for intestinal health. Soluble fibers also have a beneficial effect on blood lipid and blood sugar levels. In addition to beverages, α-cyclodextrin also lends itself to use in baker’s wares and other finished food products, because it remains stable even at high temperatures. The typical discoloration and formation of aromas in the Maillard reaction do not occur, since α-cyclodextrin contains no reducing sugars. In comparison, glucose and maltodextrin undergo significant discoloration in the reaction with the amino acid glycine (see Fig. 1.45). |
|||||||||||||||||||||
| |
|||||||||||||||||||||
 |
|||||||||||||||||||||
| Fig. 1.46: The addition of α-cyclodextrin to baker’s wares can increase the fiber content | |||||||||||||||||||||
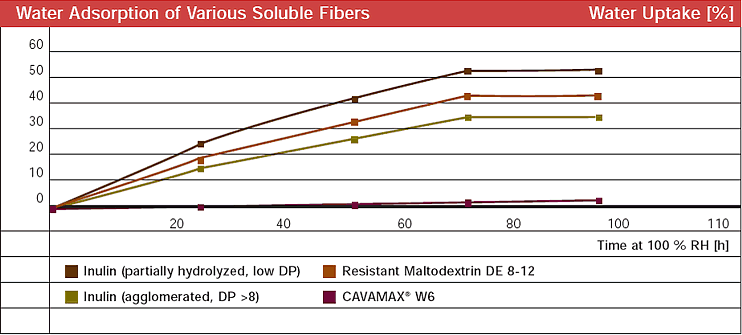
| To ensure that the baker’s wares remain fresh and crisp even during storage, the chosen added ingredients should not absorb water. Unlike other fibers such as inulin, α-cyclodextrin has very low hygroscopicity (see Fig. 1.47). | |||||||||||||||||||||
 |
|||||||||||||||||||||
| Fig. 1.47: α-Cyclodextrin has very low hygroscopicity compared with other soluble fibers | |||||||||||||||||||||
| Approval of Cyclodextrins as Food Additives | |||||||||||||||||||||
γ-Cyclodextrin with its eight glucose units is also suitable for use in the food sector. However, since food approval laws differ in each country, the use of γ-cyclodextrin is restricted to those countries where it has already been approved. All three natural cyclodextrins may already be used as food additives in the USA and Japan, whilst in Europe only β-cyclodextrin has been approved (see Table 1.8). |
|||||||||||||||||||||
|
|||||||||||||||||||||
| Table 1.8: Summary of the approval situation of cyclodextrins as food additives | |||||||||||||||||||||
Natural cyclodextrins are used as “nutraceuticals“. This term, a combination of the words “nutrition” and “pharmaceutical”, describes food additives designed to promote good health and prevent diseases caused by dietary deficiencies. Whilst α-cyclodextrin acts as a prebiotic, β-cyclodextrin and γ-cyclodextrin are used in very diverse applications due to their ability to form complexes. |
|||||||||||||||||||||
| |
|||||||||||||||||||||
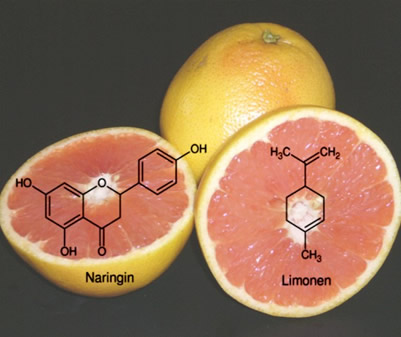
Foods containing plant extracts often have undesirable bitter tastes. Cyclodextrin molecules are able to encapsulate these components. For example, grapefruit juice is treated with cyclodextrins during preparation to remove most of the bitter-tasting naringin or limonene (see Fig. 1.48). The other flavors in grapefruits are encapsulated only to a small extent, treatment does not alter the acid and vitamin C content, so only the bitter flavors disappear. To guarantee a consistent quality, the USA sets maximum levels for the content of naringin and limonene, the two bitter-tasting components. Prior to the use of cyclodextrins in food processing, products had to be diluted with sweet juices to remain below the maximum limits. |
|||||||||||||||||||||
 |
|||||||||||||||||||||
| Fig. 1.48: Naringin and limonene are responsible for the bitter taste of grapefruit juice | |||||||||||||||||||||
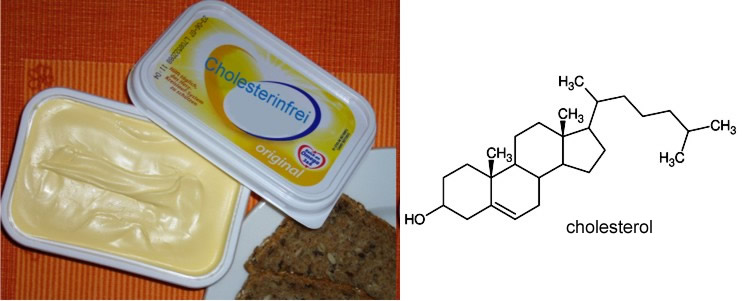
Cyclodextrin polymers are used to prevent cyclodextrins from getting into the juice. Derivatized cyclodextrin molecules are synthesized as monomer units which are cross-linked with one another. The cyclodextrin polymer, charged with naringin and limonene, is regenerated by adding sodium hydroxide solution and then washing. The ability to form host-guest complexes is also utilized in the production of cholesterol-free food. This is because a cholesterol molecule fits perfectly into a β‑cyclodextrin molecule containing seven glucose units. |
|||||||||||||||||||||
 |
|||||||||||||||||||||
| Fig. 1.49: Cholesterol-free butter or margarine is obtained by treating the fats with cyclodextrins which encapsulate the cholesterol | |||||||||||||||||||||
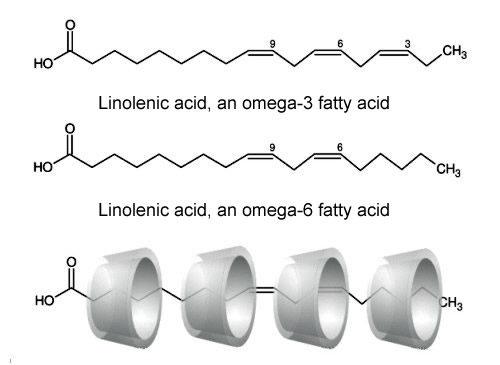
Cyclodextrin molecules are used not only in food processing, but also in food itself, where they act mainly as molecular stores. β-Cyclodextrin or γ-cyclodextrin are used most often due to their size. ω is the last letter in the Greek alphabet. For this reason, it is used to denote the carbon atom which is furthest away from the carboxyl group. The number indicates how far the first double bond lies from the last carbon atom in unsaturated fatty acids. |
|||||||||||||||||||||
 |
|||||||||||||||||||||
| Fig. 1.50: Omega-3 and omega-6 fatty acids are complexed with several cyclodextrin molecules | |||||||||||||||||||||
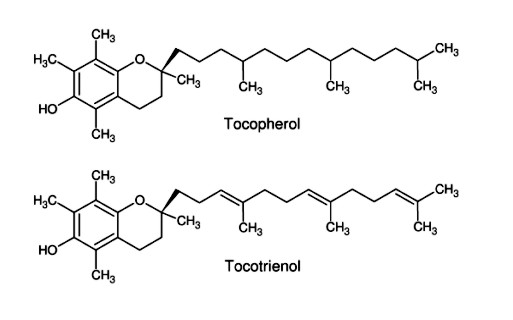
Encapsulation by cyclodextrins yields a colorless, odorless powder that is very easily processed and forms a fine suspension in water. In addition to masking odors, the host-guest complexes protect the fats from oxidation, preventing the fat from turning rancid. The cyclodextrin complex manufactured by WACKER under the brand name OmegaDry® Cranberry contains various components of cranberry seed oil encapsulated in γ-cyclodextrin. The cold-pressed cranberry seed oil contains a mixture of fats with omega-3, omega-6 and omega-9 fatty acids (see Fig. 1.50). Inclusion complexes are also formed with vitamin E (tocopherol) and the functionally isomeric tocotrienols (see Fig. 1.51). There is considerable interest in vitamin E and tocotrienol, because they are very good lipophilic antioxidants. They protect lipid molecules in the cell membranes from oxidative degradation, thus preventing premature aging of skin. They can also alleviate skin and tissue damage caused by UV radiation. Complexes with tocopherol and tocotrienol can be used in food and skin-conditioning products. |
|||||||||||||||||||||
 |
|||||||||||||||||||||
| Fig. 1.51: OmegaDry® Cranberry (WACKER product) contains cyclodextrin inclusion complexes with components from cold-pressed cranberry seed oil | |||||||||||||||||||||
|
|||||||||||||||||||||
References:
|
|||||||||||||||||||||
| | Home | Wuppertal University | WACKER | Didactic Dept. | Supp. Info | Experiments | Media | Contact | | |||||||||||||||||||||