|
4. Binding and Controlled Release of Peppermint Aroma |
||
It takes only 10 to 15 minutes to produce the complex, but the samples must be stored in the open for at least three days for a clear difference to become apparent. |
||
|
||
The results of this experiment demonstrate the properties of cyclodextrin complexes that are desirable in cosmetics, for example. The fresh peppermint aroma persists for longer due to the formation of host-guest complexes with the aroma substances in the peppermint oil, which prevent the guest molecules from evaporating so easily. The aromas encapsulated within the cyclodextrin cavities are also protected from oxidation by atmospheric oxygen. The colorless solid steadily releases aromas for a prolonged period, since the complex dissociates to a minor degree as a result of atmospheric humidity. The formation of a hydration sheath on the exterior of the cyclodextrin molecule can modify the molecular structure of the complex to such an extent that interactions between the host and guest molecules become weaker. Adding the solid to warm water facilitates dissociation of the host-guest complex due to the excess of water. An intense minty aroma is then immediately discernible, as the volatile and extremely poorly water-soluble menthol rapidly disperses in the air. |
||
For this experiment use Oleum menthae piperitae (peppermint oil), which can be obtained from the drugstore. |
||
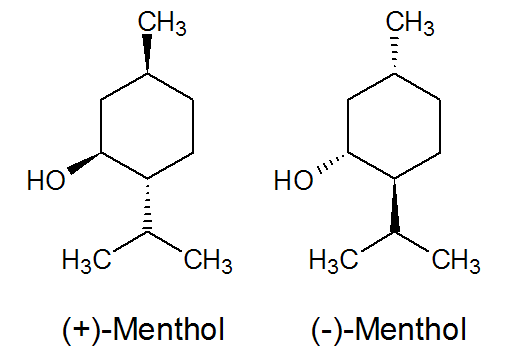
The two stereoisomers (+)-menthol and (-)-menthol are principally responsible for the peppermint aroma (see Fig. 2.25). |
||
 |
||
| Fig. 2.25: The two stereoisomers (+)-menthol and (-)-menthol cause the characteristic peppermint aroma | ||
| The menthol-cyclodextrin complex is used in cosmetics, principally because the non-polar menthol is present in the aqueous phase in the complex. The menthol-cyclodextrin complex is also used in the tobacco industry, where it is added to the tobacco in menthol cigarettes. If the menthol was not formulated as a complex, all the menthol would evaporate within a short time. | ||
|
||