 |
6. Complexation of Salicylic Acid |
 |
  1
Materials, Chemicals, Time Needed 1
Materials, Chemicals, Time Needed |
 |
- Glass beaker (50 mL, 25 mL)
- Measuring cylinder (25 mL)
- Scales
- Spatula
- Magnetic stirrer with hotplate
- Magnetic stir bar
- Filter
- Filter paper
- Drying cupboard
- Thermometer
- Oil bath
- Test tubes
- Test tube stands
- Pipette
- β-Cyclodextrin
- Distilled water
- Ethanol F, Xn
- Salicylic acid Xn
- Iron (III) chloride Xn
This experiment cannot be completed in one lesson, since the complex formed must be dried initially. Allow 20 minutes to produce the complex. When the solid obtained has dried and the oil bath has been heated to the required temperature, the tests can be performed within a few minutes during the following lesson. |
 |
  2
Procedure and Observations 2
Procedure and Observations |
 |
Dissolve 1 g β-cyclodextrin in 20 ml of a water-ethanol mixture w(ethanol) = 30%. Heat the solution to approx. 55° C whilst stirring. Then add a solution of 0.1 g salicylic acid in 2-3 ml ethanol drop by drop and stir for a further 10 minutes. Allow the solution to cool slowly to room temperature, then filter off the precipitate and wash with ethanol. Reserve a few milliliters of the filtrate for the tests. Dry the filtration residue in the drying cupboard at 30° C. Then perform the following tests on the solid obtained. Place 0.5 g of the solid in a test tube and place the test tube in an oil bath that has been preheated to 110° C. Only the lower section of the test tube should be immersed in the hot oil. For comparison, place a test tube containing 0.5 g salicylic acid in the oil bath. Observe the changes in the test tubes after a few minutes. As an additional test, add one spatula tip of
- salicylic acid,
- β-Cyclodextrin,
- Salicylic acid and β-Cyclodextrin,
- the dried compound to a separate test tube in each case,
- to another test tube add a few milliliters of the filtrate.
Add a few milliliters of the reagent solution (a dilute, weakly yellow iron (III) chloride solution) drop by drop to each test-tube. Observe the discoloration. To detect the color changes, it may be necessary to dilute the solutions with distilled water.
When the aqueous solution has cooled to room temperature, a colorless precipitate is discernible, which is powdery after drying. Although salicylic acid sublimates very readily at 110° C to form long, fine needles, no sublimation is discernible in the complex. |
 |
| Fig. 2.27: Salicylic acid sublimates very readily, whereas the complex of salicylic acid with β-cyclodextrin shows no sublimation |
 |
In the tests with iron (III) chloride, there is no decolorization with β-cyclodextrin. A violet color of varying intensity is discernible in the other tests tubes. Whilst the salicylic acid, the mixture of salicylic acid and β-cyclodextrin and the filtrate produce a strong violet color, the addition of iron (III) chloride solution to the complex produces only a weak discoloration/color change (see Fig. 2.28). |
 |
Salicylic acid |
β-Cyclodextrin |
Salicylic acid and β-Cyclodextrin |
Filtrate |
Salicylic acid- β-Cyclodextrin-complex |
|
| Fig. 2.28: The violet color change after the addition of iron (III) chloride solution is the test for salicylic acid |
 |
|
  3
Discussion of Results 3
Discussion of Results
|
 |
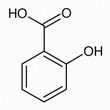
The poorly water-soluble salicylic acid is dissolved in ethanol and then added to the cyclodextrin solution. The complex forms in the solution and precipitates when the solution is cooled to room temperature. In the host-guest complex, van der Waals forces and hydrogen bonds are present between the salicylic molecule and the cyclodextrin molecule, which stabilize the complex. |
|
The complexation of salicylic acid in the cyclodextrin cavity is so stable that the characteristic properties of salicylic acid are absent. It can be shown that the salicylic acid-β-cyclodextrin complex shows no sublimation at approx. 110° C , whereas pure salicylic acid sublimates from 76° C and re-sublimates in the form of long, fine needles in the upper part of the test tube (see Fig. 2.27). |
|
| Fig. 2.29: Van der Waals forces and hydrogen bonds retain the salicylic acid inside the cyclodextrin molecule |
 |
Iron (III) chloride is used to detect salicylic acid, because the salicylic acid (2-hydroxybenzoic acid) forms violet colored salts with iron (III) chloride. In the test with the salicylic acid-β-cyclodextrin complex, although the violet color is not completely absent, it is much fainter than with free salicylic acid. There are two reasons for this: Minute quantities of non-complexated salicylic acid could still be present in the solid, or the addition of the aqueous iron (III) chloride solution could trigger the dissociation of the complex, producing a positive result in the detection reaction. Both tests must be performed to conclusively prove the formation of a salicylic acid-β-cyclodextrin complex. The absence of sublimation could also indicate that the solid contains no salicylic acid. However, the reaction with iron (III) chloride is a clear test for salicylic acid, since with β-cyclodextrin, the addition of iron (III) chloride solution produces no violet coloration. The reaction with iron (III) chloride alone would be insufficient, since salicylic acid could also be free in the β-cyclodextrin mixture. |
 |
  4
Tips and Comments 4
Tips and Comments
|
 |
| Instead of using simple filtration, the solid could also be obtained more quickly with a glass filtering crucible and water jet pump. This produces a much drier complex. |
 |
  5 Supplementary Information 5 Supplementary Information |
 |
The tests must be performed with the dried complex, since there is always an equilibrium between the complex and the dissociated molecules in the presence of water, and so the formation of a complex cannot be detected. |
 |
  6 References 6 References |
 |
- Woyke, A.; „Cyclodextrine“ – Molekulare Zuckertüten. Ein Chemie-Praktikum für die 13.Klasse (www.science-forum.de/download/Cyclo-praktikum.pdf)
- Römpp Online Chemielexikon Version 2.13; Stichwort „Salicylsäure“
- Becker, H.G.O.; Organikum; Wiley-VCH Weinheim, 2001, S. 548
|
 |
  | Home | Wuppertal University | WACKER | Didactic Dept. | Supp. Info | Experiments | Media | Contact | | Home | Wuppertal University | WACKER | Didactic Dept. | Supp. Info | Experiments | Media | Contact | |


