|
This experiment takes only a few minutes to prepare. The samples need stirring for 20 minutes to obtain clear observations. |
||
|
||
Naringin and limonene are the substances responsible for the extremely acidic smell of grapefruit and in particular the bitter taste. When the juice is treated with β-cyclodextrin, these two substances are largely encapsulated inside the cyclodextrin molecules. As with masking the smell of garlic, complexes with naringin and limonene are low-odor, so the acidic smell of the grapefruit juice disappears. |
||
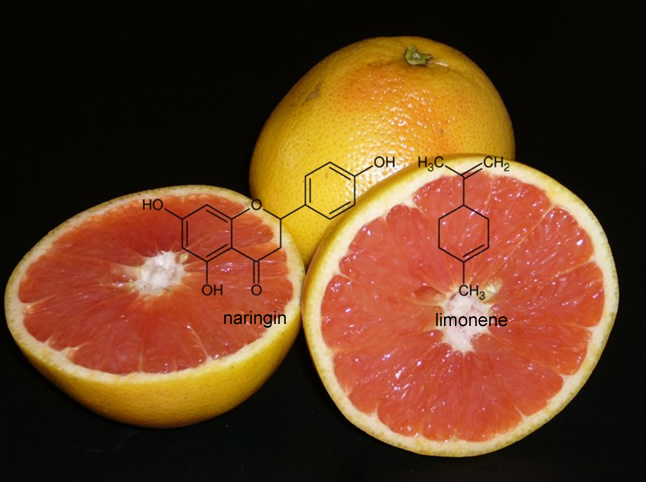 |
||
| Fig. 2.26: The bitter taste of grapefruit juice is removed by the complexation of naringin and limonene | ||
Since tasting samples is not permitted in chemistry lessons, use your sense of smell to confirm the change produced by treating with cyclodextrins! For a reliable test of the effect of β-cyclodextrin on the grapefruit juice, the sample should be blind tested i.e. students should not know which sample contains the cyclodextrin. The students then describe the smell of the samples and compare their findings. Use freshly squeezed grapefruit juice for this experiment, because the acidic note in shop-bought juice is too weak to show a clear effect of the addition of cyclodextrins. |
||
The complexation of bitter principles in grapefruit juice is used in food processing to reduce the extremely acidic taste. This is not done using water-soluble cyclodextrins, however, since the cyclodextrin complexes formed would then have to be removed from the juice in a further production stage. To simplify the treatment, the juice is passed through a cyclodextrin polymer. |
||
|
||