|
If the solutions are already prepared, the experiment can be performed in 10 – 15 minutes. Follow-up experiments are also available (Substitution of the Guest Molecule and Dissociation of the Complex). |
||||
|
||||
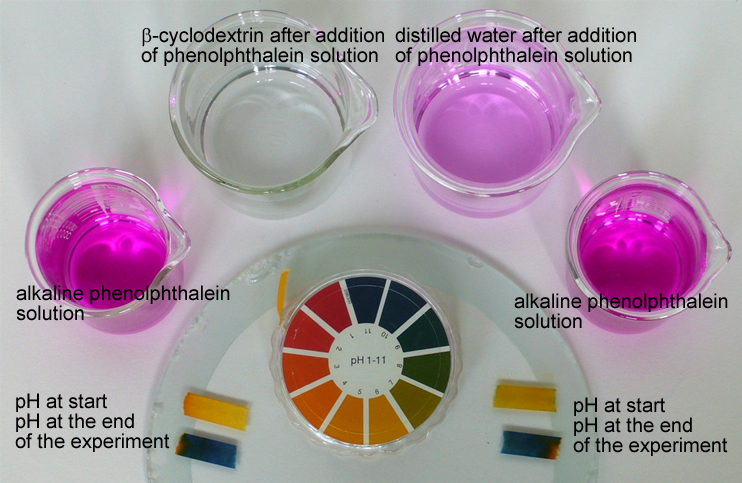
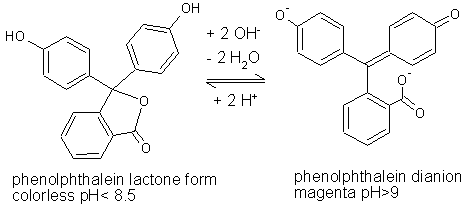
The decolorization of the phenolphthalein solution in the two variants cannot be attributed to the shift in pH towards the acid range, since the suspension or solution is demonstrably alkaline (pH = 10 – 11). When the magenta phenolphthalein solution is added to β-cyclodextrin or to the β-cyclodextrin solution, the cone-shaped β-cyclodextrin forms a host-guest complex with the guest molecule phenolphthalein, due to van der Waals interactions. Since the molecular structure is non-ionic and therefore less polar in acid and neutral conditions, it can be assumed that the phenolphthalein is complexated in this form, which would also explain the decolorization of the solution. |
||||
 |
||||
| Fig. 2.14: Molecular structures of phenolphthalein at pH < 8.5 and pH > 9 | ||||
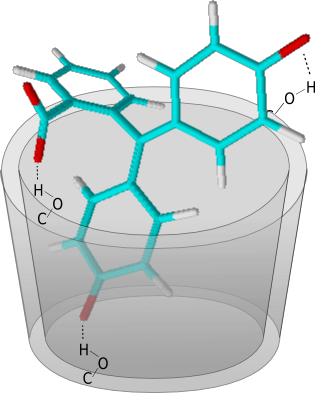
UV-VIS spectroscopy of different substances and complexes has shown that the phenolphthalein is present in the cyclodextrin in the dianionic form. During the formation of the host-guest complex, the phenolphthalein dianion is complexated by the formation of three hydrogen bonds to the cyclodextrin molecule. Therefore the van der Waals forces between the guest molecule and the non-polar cavity of the cyclodextrin are not the significant forces in this complex. |
||||
 |
||||
| Fig. 2.15: Schematic diagram of the hydrogen bonds between the β-cyclodextrin molecule and the phenolphthalein dianion | ||||
These intermolecular interactions cause the phenolphthalein molecule to twist more strongly around the central carbon atom. Delocalization of the conjugated π-electron is consequently impaired, so that the color disappears. |
||||
The observation that phenolphthalein decolorizes in the presence of cyclodextrin despite the alkaline solution forms the basis of one analytical method for determining the cyclodextrin content. In UV-VIS spectra, the cyclodextrin content can be determined by the absorption at 550 nm, where the phenolphthalein dianion has the most intense absorption band.
Further experiments also use the decolorization of the phenolphthalein solution to test for cyclodextrins. For instance, this simple experiment (Extraction from Textile Fresheners and Detection of Cyclodextrins) can be used to test textile fresheners for cyclodextrins. Cotton fabric finished with cyclodextrins is also tested in this way (Cyclodextrins in Textile Finishing). If the red-violet solution loses its color even when the fabric strip has been washed in water, then it is certain that the cyclodextrin molecules are bonded covalently to the cotton fiber via the anchor molecule. |
||||
|
||||