|
7. Cyclodextrins in Textile Finishing |
||||
Allow 10 minutes to make the solution for treating the cotton strips. Treating the cotton strips and testing for successful textile finishing with cyclodextrins takes a further 10 minutes. |
||||
|
||||
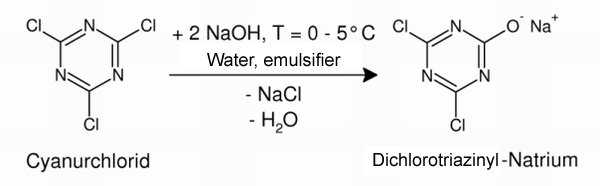
Since β-cyclodextrin is insufficiently reactive to bond directly to the cotton fiber, the cyclodextrin is first derivatized. The reactive cyanuric chloride, which is initially converted using sodium hydroxide solution, serves as an anchor molecule. In the reaction the hydroxide ion attacks a positively polarized carbon atom in the cyanuric chloride. The resulting dichlorotriazinyl-sodium is considerably more water-soluble. |
||||
 |
||||
| Fig. 2.31: Cyanuric chloride is converted to dichlorotriazinyl-sodium salt before the reaction with β-cyclodextrin | ||||
| Under alkaline conditions a second chlorine atom is substituted by a β-cyclodextrin molecule. | ||||
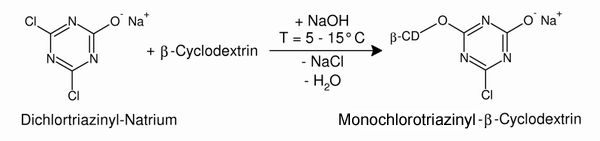 |
||||
| Fig. 2.32: The monochlorotriazinyl-β-cyclodextrin is sufficiently reactive to react with hydroxyl groups in the cotton fiber. | ||||
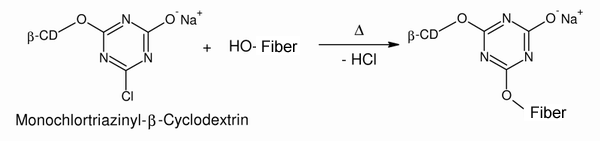
| The chlorine-substituted carbon atom in the monochlorotriazinyl-β-cyclodextrin is so strongly positively polarized that nucleophilic substitution with a hydroxyl group from the cellulose occurs. | ||||
 |
||||
| Fig. 2.33: The covalent bonding of the cyclodextrin derivative to the cellulose is effected by means of heating, during which hydrogen chloride is split off | ||||
The test cannot be performed until the non-bonded cyclodextrin has been washed out of the cotton strips. If the textile surface is finished with cyclodextrin molecules, the alkaline phenolphthalein solution decolorizes immediately, due to the formation of a colorless cyclodextrin complex with the phenolphthalein dianion. |
||||
When the soaked cotton strips are heated between the hotplate and the oil bath, they may turn brown due to the thermal decomposition of the cyclodextrin. In this case heating must be reduced. The hissing sound caused by water evaporation provides a clue. When most of the water has evaporated, the hissing stops and the fabric sample can be removed from the aluminum foil. Heat treatment is required to fix the cyclodextrin derivative to the cotton surface. If a cotton sample soaked in the cyclodextrin solution is rinsed in water and the test with phenolphthalein solution is subsequently performed, the red-violet color remains visible. The washing stage following heat treatment is also essential. This ensures that any unfixed cyclodextrin is washed out of the cotton strips, and therefore cannot falsify the phenolphthalein test results. The finished cotton strips can also be used in the experiment for masking the smell of cigarette smoke, and tested for functionality. |
||||
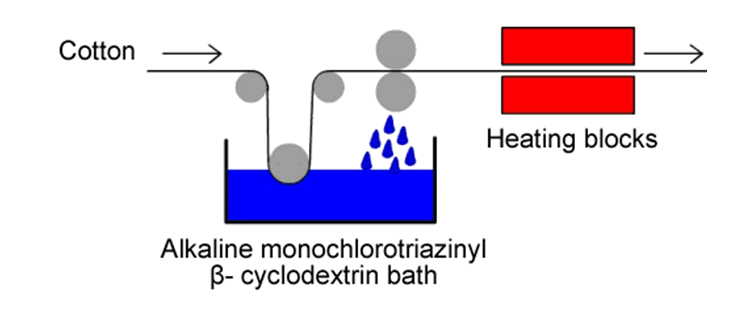
In industry, cotton is finished with cyclodextrins in a continuous process (see Fig. 2.34). After the fabric panel has been fixed, the fabric is washed in water and then dried. |
||||
 |
||||
| Fig. 2.34: Continuous process of cotton finishing with cyclodextrins | ||||
| Functionalized textiles are used to mask unpleasant odors such as smoke or perspiration. Textiles can also be loaded with specific fragrances or skin-conditioning substances, which are then steadily released when the garments are worn. Washing removes the guest molecules from the cyclodextrin molecules, leaving the cyclodextrin molecules free to absorb more odors, fragrances or other substances. | ||||
|
||||
