|
7. Substitution of the Guest Molecule in the β-Cyclodextrin-Phenolphthalein Complex |
 |
  1
Materials, Chemicals, Time Needed 1
Materials, Chemicals, Time Needed |
 |
- Pipette
- Test tube with bung or snap-cap vial
- (Test tube stands)
- β-Cyclodextrinp henolphthalein solution from the previous experiment
- Benzyl alcohol Xn
This experiment only takes one minute, but the previous experiment Host-Guest Complex: β-Cyclodextrin and Phenolphthalein must be performed first by way of preparation. |
 |
  2
Procedure and Observations 2
Procedure and Observations |
 |
|
  3
Discussion of Results 3
Discussion of Results
|
 |
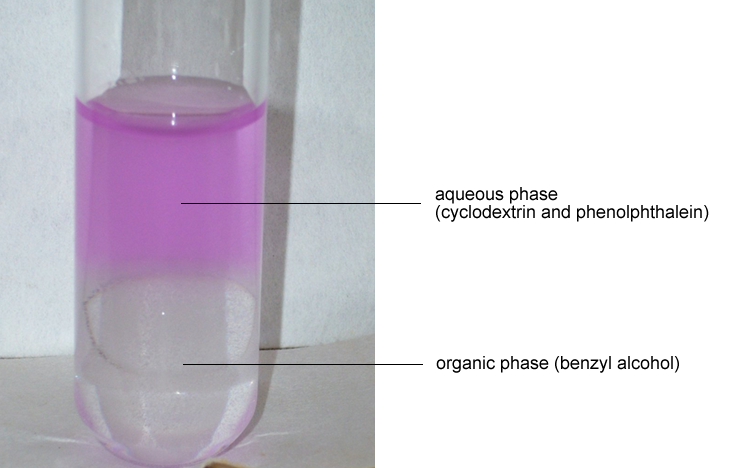
| Shaking the β-cyclodextrin-phenolphthalein complex in the presence of benzyl alcohol results in the partial substitution of phenolphthalein by benzyl alcohol. |
 |
| Abb. 2.17: Benzyl alcohol |
 |
The phenolphthalein molecules expelled from the cyclodextrin molecules are responsible for the pink color. This is because expulsion from cyclodextrin breaks the hydrogen bonds between the phenolphthalein and the cyclodextrin molecule, enabling the molecule to reposition itself so that the delocalization of the conjugated π-electron in the phenolphthalein dianion is improved. This shifts absorption to the visible range and the characteristic red-violet color of phenolphthalein then appears in the alkaline solution. |
 |
  4
Tips and Comments 4
Tips and Comments
|
 |
  5 Supplementary Information 5 Supplementary Information |
 |
This guest substitution in the host-guest complex can be used to determine which molecules form a complex with cyclodextrin. Comparison of the complex formation constants of the cyclodextrin complex with benzyl alcohol and phenolphthalein (Tab. 2.4) leads to the hypothesis that the phenolphthalein complex should remain. But in fact the equilibrium shifts in favor of the β-cyclodextrin-benzyl alcohol complex, due to the addition of an excessive quantity of benzyl alcohol (conc. of benzyl alcohol/conc. phenolphthalein ≈ 2 • 104). Consequently the phenolphthalein is replaced by benzyl alcohol, the new guest component. The fact that the expelled phenolphthalein dianion can be easily hydrated, whereas the benzyl alcohol is poorly water soluble further facilitates this guest substitution.
|
 |
Definition of the complex formation constants |
β-Cyclodextrin complex with phenolphthalein |
β-Cyclodextrin complex with benzyl alcohol |

|
23 · 103 |
21 |
|
 |
| Tab. 2.4: Complex formation constants for the β-Cyclodextrin complexes with phenolphthalein and benzyl alcohol |
 |
  6 References 6 References |
 |
- Tausch, M.; von Wachtendonk, M.; Chemie 2000+ Band 3, C.C. Buchners Verlag Bamberg, 2005, S. 26
- Buvári, A.; Barcza, L.; J. Chem. Soc. Perkin Trans. II, 1988, 1687-1690
- Szejtli, J.; Osa, T.; Comprehensive Supramolecular Chemistry. Volume 3 Cyclodextrins, Elsevier Science Ltd. Oxford, 1996, S. 206
- Rekharsky, M. V.; Inoue, Y.; Chem. Rev., 1998, 98, 1875-1917
|
 |
  | Home | Wuppertal University | WACKER | Didactic Dept. | Supp. Info | Experiments | Media | Contact | | Home | Wuppertal University | WACKER | Didactic Dept. | Supp. Info | Experiments | Media | Contact | |