|
8. Dissociation of the β-Cyclodextrin-Phenolphthalein Complex |
||
This experiment can be performed in 2 minutes if you have already done the experiment to form the host-guest complex from β-cyclodextrin and phenolphthalein. |
||
|
||
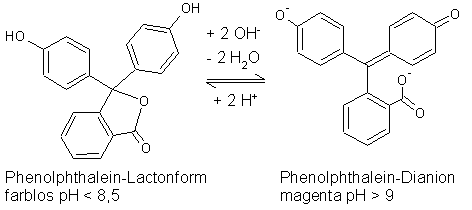
In this experiment, too, the existing equilibrium can be altered by modifying the concentration of hydroxide ions. The modified conditions compel some of the encapsulated phenolphthalein dianions to leave the host cyclodextrin. Discoloration of the solution can also be explained by looking at the equilibrium between the phenolphthalein dianion and the phenolphthalein lactone form. |
||
 |
||
| Fig. 2.18: Molecular structures of phenolphthalein at pH < 8.5 and pH > 9 | ||
If the equilibrium at pH > 9 is not completely on the side of the dianion, the addition of hydroxide ions causes the equilibrium to shift to the right. If all the cyclodextrin molecules are already occupied by guest molecules, then the dianionic form is present in the solution, which causes the red-violet color observed in the solution. |
||