  Pyrogenic
Silica for Thixotropic Liquids Pyrogenic
Silica for Thixotropic Liquids
What have pyrogenic silicas (HDK®)
to do with silicones? Well, for one thing, HDK® is
used for precisely adjusting the desired properties of silicones
(see below). For another, the production of these products
is closely linked to the Manufacture
of silicones at WACKER. When
chlorosilanes (tetrachlorosilane and
others), which are essentially unwanted volatile by-products
of the silicones
production process, are introduced into a hydrogen flame, they
react with water formed in situ to
produce pyrogenic silica (HDK®),
whose composition approximates to SiO2:
SiCl4 + 2H2 + O2 ® SiO2 +
4HCl
A carefully controlled process and short contact time in the flame
ensures that the silica is produced in the form of nano-particles.
Since this synthetic silica is formed in a flame and is a white powder,
it is also referred to as white carbon black. Its configuration and
properties are discussed in detail below.
First, though, let us explain what a thixotropic material is:
 |
Silicone rubber, which is used for insulating
metal wires and glass fibers, may contain up to 40 % pyrogenic silica.
Here, the HDK® ensures that the vulcanized silicone
rubber becomes an elastic material that has excellent mechanical
properties.
The flow properties of paints and coatings can be controlled precisely with pyrogenic silica. HDK® is accordingly used in modern paints and coatings as a rheological additive. |
 |
  HDK® is used as an active filler in joint sealants and as a free-flow agent in fire extinguisher powders. In toothpastes, its function is to impart the desired consistency. HDK® is used as an active filler in joint sealants and as a free-flow agent in fire extinguisher powders. In toothpastes, its function is to impart the desired consistency. |
 |
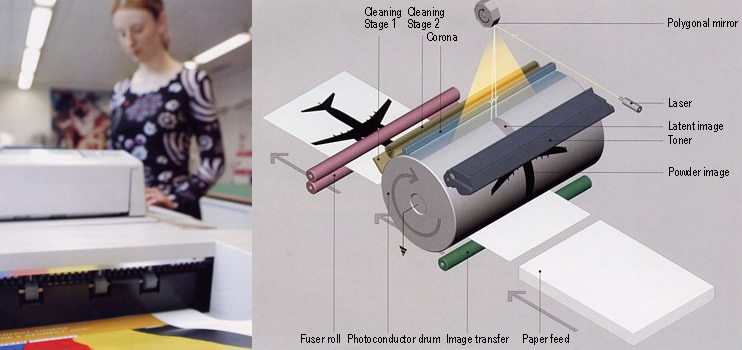
| In modern laser printers, a latent, invisible image is first written with light on an electrically charged photoconductor. The toner powder particles must “sense” the charge differences in the latent image, such that they are deposited in certain places but not in others. The result is the powder image, which, in a subsequent step, is transferred to the paper and then fixed by fusing. Strict demands are made on the toner in respect of its free-flow quality and its electrical and thermal properties. Small additions of HDK® pyrogenic silica to the toner make it possible to regulate its free-flow property, so that HDK® effectively “fine-tunes” the toner in laser printers. |
 |
 |
  Structure
and Properties of Pyrogenic Silica Structure
and Properties of Pyrogenic Silica |
 |
| Pyrogenic silica is a very efficient thixotropic
agent. This property is based on its structural characteristics.
Although it has essentially the same composition as sand and
quartz , structural differences exist. The following model of
the atomic lattice of silica shows that every silicon atom is
surrounded by four covalently bonded oxygen atoms: |
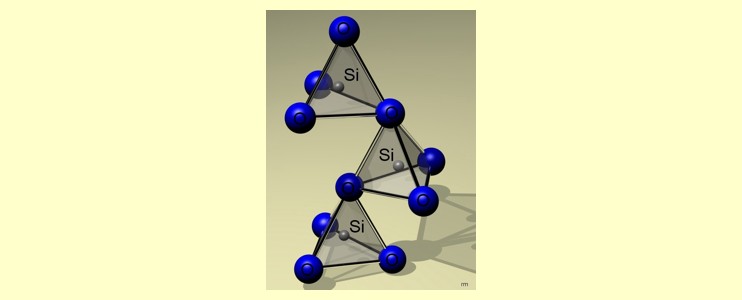 |
  In
sand and quartz, these SiO4-SiO4 tetrahedra
are regularly arranged in a three-dimensional crystal lattice,
but in a primary
particle of pyrogenic silica they form a disordered amorphous system.
A primary particle contains about 10.000 SiO2 units
and is a so-called nano-particle with a diameter between 5 and
30 nm (1 nm = 10-9 m).
The outwardly projecting oxygen atoms are bonded to hydrogen
atoms. So a primary particle is like a hedgehog whose spines
consist of -OH groups These groups render the surface of a primary
particle hydrophilic because they can form hydrogen bonds with
water molecules. However, they also cause many stationary primary
particles to form extensive three-dimensional networks through
aggregation (aggregates of nano-particles). In
sand and quartz, these SiO4-SiO4 tetrahedra
are regularly arranged in a three-dimensional crystal lattice,
but in a primary
particle of pyrogenic silica they form a disordered amorphous system.
A primary particle contains about 10.000 SiO2 units
and is a so-called nano-particle with a diameter between 5 and
30 nm (1 nm = 10-9 m).
The outwardly projecting oxygen atoms are bonded to hydrogen
atoms. So a primary particle is like a hedgehog whose spines
consist of -OH groups These groups render the surface of a primary
particle hydrophilic because they can form hydrogen bonds with
water molecules. However, they also cause many stationary primary
particles to form extensive three-dimensional networks through
aggregation (aggregates of nano-particles). |
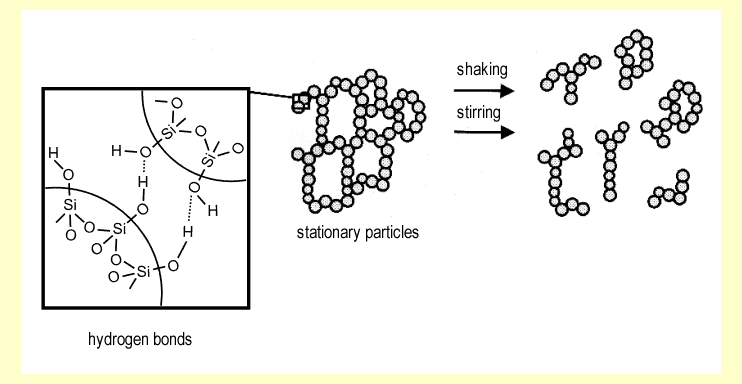 |
| Fluids or gases can be stored and largely
immobilized in the interstitial space of these aggregates. If
the liquid is water or an aqueous solution, hydrogen bonds are
formed between the water molecules and the -OH groups on the
surface of the silica particles. In this state, the system is
viscous to solid on a macroscopic scale. Mechanical stress, such
as shaking or stirring, temporarily disturbs the silica nano-particle
aggregates. This ruptures some of the hydrogen bonds holding
the particles together. Macroscopically, the system becomes free-flowing
(thixotropic effect). The longer the external force acts, the
more bonds are ruptured and the "more fluid" the material
becomes. When the force stops, three-dimensional aggregates are
re-formed and the material becomes viscous to solid again (see
diagram above). |
|
|