1. Experiment is contained in the WACKER's Experimental Kit. |
No |
2. Experimental procedure has been modified |
/ |
3. A separate experimental procedure has been devised |
Yes |
4. Video clip available |
No |
5. Flash animation available |
No |
6. Other materials: Slide
B4 |
 |
Synthesis of Tetrachlorosilane |
 |
  1
Materials, Chemicals, Time Needed 1
Materials, Chemicals, Time Needed |
 |
- 2 retort stands with connecting rod
- Retort clamps and sleeves
- Thick-walled reaction tube
- Dropping funnel with pressure compensation,
stopper, small two-neck round-bottom flask
- 3 test tubes with
side arms
- 2 absorption bottles
- Liebig condenser (with readily cleaned
inner tube) and tubing
- Tall, large glass beaker
- Three-way valve
- Perforated rubber stoppers
- Angled glass tubes (see sketch)
- Straight glass connecting tubes
- Tubing connectors
- Magnesia or porcelain boat
- Teclu burner
- Hydrochloric acid, conc, w = 37%, C
- Sulfuric acid, conc, w
= 98 %, C
- Sodium hydroxide, conc, w = 30 %, C
- Potassium permanganate, O, Xn
- Silicon powder
- Sodium chloride for freezing mixture
- Sodium hydrogen sulfite, Xi
This experiment requires
a lot of time. Overall, one repetition will take from 1.5 to
2 hours. It takes 30 to 60 minutes just for set-up, the precise
time depending on experience and how well the glassware fits
together. The experiment itself takes 20 to 30 minutes. Allow
an additional 30 minutes for dismantling and cleaning the apparatus. |
 |
  2
Procedure and Observations 2
Procedure and Observations |
 |
As chlorine will be used,
conduct the experiment in a fume cupboard. Also use the fume
cupboard when flushing the apparatus for the first time.
Wear safety glasses, rubber gloves and laboratory coat.
Set up the apparatus as shown in
the diagram. |
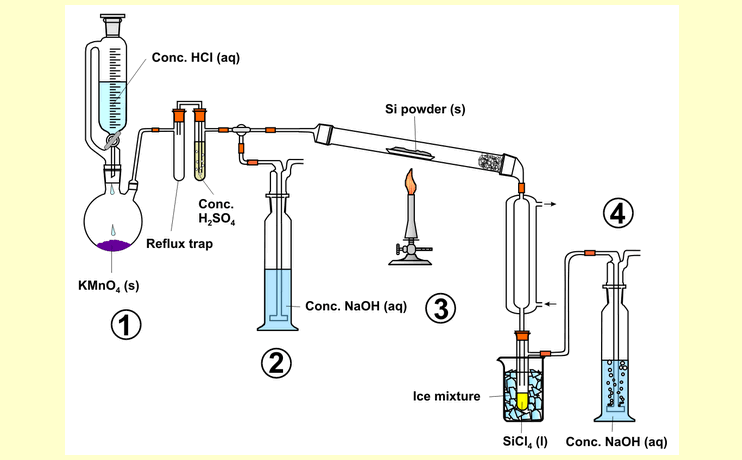 |
  Place
about 5 g potassium permanganate in the round-bottom flask and
add 40 ml concentrated hydrochloric acid to the dropping funnel.
To the drying and absorption train, add the sulfuric acid for
drying the chlorine gas, and the sodium hydroxide solution for
absorbing it. Place the amorphous silicon powder in a porcelain
or magnesia boat in the center of the reaction tube. Place
about 5 g potassium permanganate in the round-bottom flask and
add 40 ml concentrated hydrochloric acid to the dropping funnel.
To the drying and absorption train, add the sulfuric acid for
drying the chlorine gas, and the sodium hydroxide solution for
absorbing it. Place the amorphous silicon powder in a porcelain
or magnesia boat in the center of the reaction tube.
Assemble the complete apparatus and test it for leaks by connecting
the gas generator to the reaction tube via the three-way valve
and warming the flask with your hand. You should notice a pressure
difference in the second absorption bottle.
Cool the receiving vessel with the freezing mixture and turn
on the cooling water. Now open the three-way valve of the dropping
funnel in the direction of the first absorption bottle and allow
hydrochloric acid to drip slowly onto the potassium permanganate.
When the chlorine gas is being generated at a uniform rate, switch
over the three-way valve and flush the rest of the apparatus
with chlorine before using the Teclu burner to heat the silicon
in the reaction tube, gently at first and then strongly. When
about 0.5 to 1.0 ml product has collected in the receiving vessel,
cool the reaction tube in the stream of chlorine initially. Then
switch the three-way valve over to the first absorption bottle.
The receiving vessel can now be removed and the product used
for other purposes.
When the hydrochloric acid drips onto the potassium permanganate,
puffs of greenish chlorine gas are initially evolved with every
drop. After about 1 minute, the gas is evolved at a uniform rate;
this can be seen from the bubbles rising in the sulfuric acid.
When heated in the stream of chlorine gas, the silicon powder
in the boat lights up brightly in a small line following prolonged
strong heating on the side nearer the dropping funnel. The glow
front gradually follows the direction of the gas stream. White
smoke forms behind the silicon and precipitates out on the rear
section of the tube in the form of a yellow to orange coating.
Clear condensation is observed in the upper section of the Liebig
condenser. A yellow-orange liquid slowly collects in the receiving
vessel. During this reaction, no bubbles are seen in the absorption
bottle at the end of the apparatus.
Stop heat input after about 0.5 ml product has formed. Smoke
formation ceases very quickly. After a short interval, small
bubbles may be seen forming again in the absorption bottle. When
the reaction tube is cool enough to be handled, turn off the
supply of hydrochloric acid and direct the chlorine gas into
the first absorption bottle. When no more chlorine gas is being
generated, open the apparatus inside the fume cupboard and leave
for one hour. Then clean it.
Keep the tetrachlorosilane for another experiment (see "Hydrolysis
of tetrachlorosilane”). |
 |
  3
Discussion of Results 3
Discussion of Results
|
 |
At elevated temperatures, silicon
reacts with chlorine to form tetrachlorosilane.

This is a redox reaction in which the silicon is oxidized by the chlorine.
Although the reaction is exothermic, it only proceeds quickly at elevated
temperatures. Since only a relatively small amount of silicon is used in
the experiment, the heat of reaction is not enough to keep the reaction
going. For this reason, continued heating is needed while the chlorine
is reacting with the silicon.
The silicon sample is not fully consumed in the reaction, but its appearance
is substantially altered by the reaction. The following photomicrographs
show the sample before and after use in this experiment:

The mass of the solid reagent decreases by several hundred
milligrams during the reaction with chlorine. There is little
point in trying to quantify the reaction exactly because,
firstly, the change in weight is too slight and, secondly,
the silicon sample used generally still contains traces of
aluminum and iron, which also react with chlorine. |
 |
 |
The AlCl3 and
FeCl3 formed sublime relatively easily and are
deposited as a yellow (pure aluminum chloride), but more
often orange (aluminum chloride contaminated with iron
chloride) solid on the wall of the reaction tube or the
condenser.
Tetrachlorosilane is a clear, water-white liquid when pure,
but it is obtained in this experiment as a yellow to orange
liquid due to the contaminants just mentioned. The reaction
product shown in the photo is a fairly heavily contaminated
sample of tetrachlorosilane.
|
 |
|
 |
  4
Tips and Comments 4
Tips and Comments
|
 |
- If a Liebig condenser is not
used, it could easily happen that the freezing mixture
is not cold enough to cool the product and that the product
will be
driven into the absorption bottle. There, it forms a layer
of foam on the surface of the sodium hydroxide. This will
not occur if the stream of chlorine gas is very weak, but that
is very hard to achieve.
- Try to prevent too much deposition on the reaction tube
because that only further contaminates the product.
- A Liebig condenser with a larger internal diameter will
make it easier to brush clean since some of the deposit there
is not
immediately soluble in water. A condenser of this kind provides
adequate cooling since no observations to the contrary are
made in the absorption bottle.
- The pressure equalizer can be easily cleaned of manganese
dioxide with a weakly acidic, highly dilute solution of sodium
hydrogen
sulfite. Since this generates sulfur dioxide, it is imperative
that cleaning be performed in the fume cupboard.
- In the References quoted below, this experiment
is included as a teacher’s experiment in the lesson entitled
manufacture and properties of silicon.
|
 |
  5
Supplementary Information 5
Supplementary Information
|
 |
The example of tetrachlorosilane
clearly shows that silicon occupies an intermediate position
between metals and non-metals.
On one hand, tetrachlorosilane is not a typical salt like the
metal chlorides, but rather a liquid like carbon tetrachloride,
i.e. a chloride of carbon, a typical non-metal. On the other
hand, the reaction between tetrachlorosilane and water is different
from that of carbon tetrachloride and water. Its reaction with
water resembles those of the metal chlorides SnCl4 and AlCl3 (see also the experiment “Hydrolysis
of tetrachlorosilane”. |
 |
  6
References 6
References |
 |
| M. Tausch, M. von Wachtendonk
(editors), CHEMIE S II, STOFF-FORMEL-UMWELT, C.C. Buchner, Bamberg
(1993), (1998), S. 357f |
 |
|