Textile fresheners contain cyclodextrins which remove the smell of cigarette smoke, cooking, pets and perspiration when the fresheners are sprayed onto textiles. |
|
 |
|
| Fig. 1.31: Most textile fresheners contain cyclodextrins |
Fig. 1.32: To prevent the formation of odors as they develop, cyclodextrins must bond to the textile fibers |
Fresheners can also be used to mask food smells in curtains, unpleasant odors in clothing, sofas and armchairs, and the smell of perspiration in sports textiles. |
|
| |
|
| To make the odor-absorbing property of cyclodextrins permanent in textiles, the cyclodextrin molecules must be anchored to the textile fibers to prevent them from being washed out. | |
 |
|
| Fig. 1.33: WACKER markets innovative products for functional textile finishing with cyclodextrins under the name CAVATEX® | |
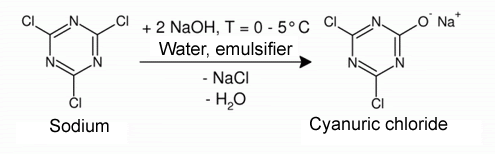
Since natural cyclodextrins cannot bond directly to the textile surface, the cyclodextrins are first functionalized. Cyanuric chloride, which initially reacts to form a dichlorotriazinyl sodium salt in alkaline conditions, serves as the anchor molecule for the covalent bond to the cellulose fiber (see Fig. 1.). |
|
 |
|
| Fig. 1.34: Cyanuric chloride is converted to the dichlorotriazinyl sodium salt before the reaction with β-cyclodextrin | |
| |
|
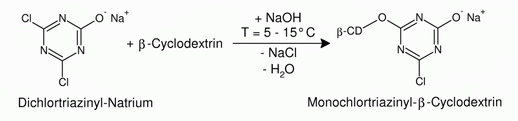 |
|
| Fig. 1.35: A high yield of monochlorotriazinyl-β-cyclodextrin, the reactive derivative of β-cyclodextrin, is obtained in a one-pot reaction. | |
| The C-Cl bond in the monochlorotriazinyl-β-cyclodextrin is so strongly polarized that the carbon atom from a cellulose molecule can be attacked by an oxygen atom from a hydroxyl group. | |
 |
|
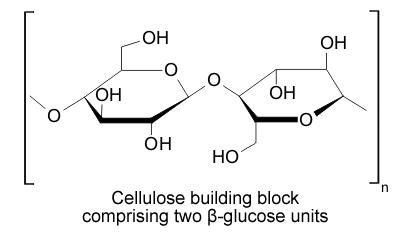
| Fig. 1.36: Section of a cellulose molecule | |
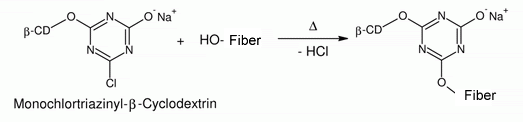
| In this way, the anchor molecule helps to bond the β-cyclodextrin to the cellulose fiber (see Fig. 1.37). | |
 |
|
| Fig. 1.37: The covalent bond to the cellulose fiber is effected in a condensation reaction during which hydrogen chloride is split off | |
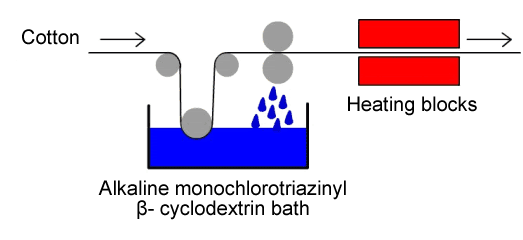 |
|
| Fig. 1.38: In industry, cotton finishing with monochlorotriazinyl-β-cyclodextrin is performed in a continuous process | |
The German Center for Textile Research for the Northwest (Deutschen Textilforschungszentrum Nord-West) is researching and testing textile finishing methods based on cyclodextrin. To quantify the functionalization of a textile surface with cyclodextrins, the researchers have developed an analytical method which exploits the ability of cyclodextrins to form complexes. |
|
 |
|
| Abb. 1.39: The German Centre for Textile Research for the Northwest (deutsches Textilforschungszentrum Nord-West) is developing and testing methods for textile finishing based on cyclodextrin | |
|
|
 |
|
| Fig. 1.40: When an alkaline phenolphthalein solution is pipetted onto a treated (left) and untreated (right) cotton strip, the ensuing color loss is proof of textile finishing with cyclodextrins | |
Textiles finished in this way can absorb unpleasant odors, such as those from cigarette smoke or pets, by forming host-guest complexes between the cyclodextrin molecules and the odor molecules. |
|
 |
|
| Fig. 1.41: Men's suits finished with cyclodextrins were on the market as early as 2001 | |
Complexation of essential sweat components can suppress microbiological processes and prevent the smell of sweat from occurring at all . Washing removes the encapsulated compounds from the cyclodextrin molecules, leaving the anchored cyclodextrin molecules free to absorb more odors. |
|
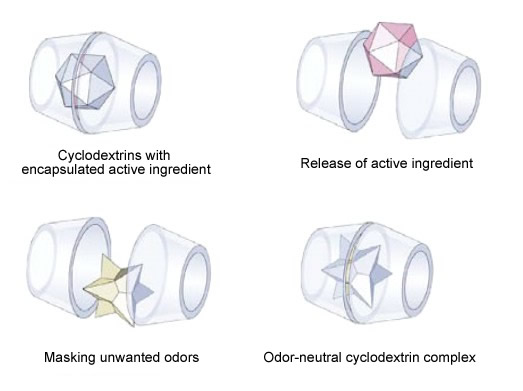 |
|
| Fig. 1.42: Schematic diagram showing the mode of action during the masking of unwanted odors and the release of an encapsulated active ingredient | |
The cyclodextrins anchored to the textile fibers can also be loaded with specific fragrant or skin-conditioning substances. As moisture triggers the dissociation of these host-guest complexes, the guest molecules are only released through perspiration or when drying oneself with a treated hand towel. This enables the controlled release of the guest components. |
|
 |
|
| Fig. 1.43: Fragrances are released from the cyclodextrin molecules only when the bear is touched | |
References:
|
|
| | Home | Wuppertal University | WACKER | Didactic Dept. | Supp. Info | Experiments | Media | Contact | | |