 |
||||||||||||||||||||
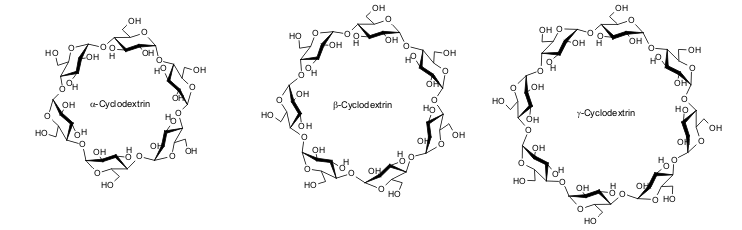
| Fig. 1.15: Molecular structure of the three natural cyclodextrins | ||||||||||||||||||||
The considerable interest in cyclic oligosaccharides can be attributed to the unusual architecture of the molecular structure of cyclodextrins. Mathematically, the three-dimensional shape can be described as an open truncated cone. It is also sometimes described as a “molecular candy cone” to reflect the fact that the cyclodextrin molecule is composed of several glucose units. Another image conjured up by the phrase “the world's smallest beauty case” is a reference to the use of cyclodextrins in cosmetics. |
||||||||||||||||||||
|
||||||||||||||||||||
| Table 1.2: Dimensions of cyclodextrin molecule cavities | ||||||||||||||||||||
| |
||||||||||||||||||||
 |
||||||||||||||||||||
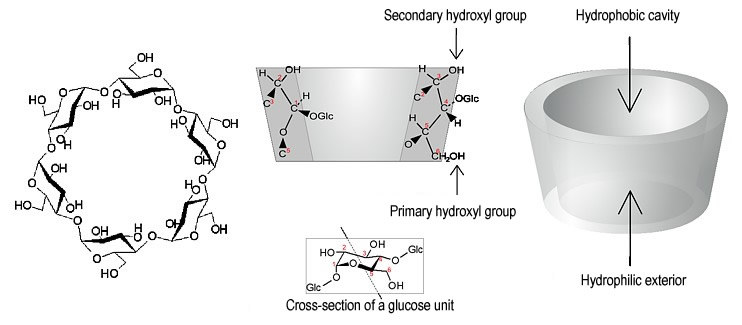
| Fig. 1.16: Molecular structure of β-cyclodextrin, cross-section of a cyclodextrin molecule showing the arrangement of a glucose unit and conical representation showing hydrophilic exterior and hydrophobic cavity. | ||||||||||||||||||||
The spatial structure of cyclodextrin becomes far clearer when observed as a three-dimensional, animated molecular model. Fig. 1.16 shows that the hydroxyl groups are located on the upper and lower edges of the cyclodextrin molecule. Only the hydrogen atoms at carbon atoms 3 and 5 (see Fig. 1.16) and the oxygen atoms in the glycosidic bond between the two glucose units (see Fig. 1.17) project inwards. As there are no protic hydrogen atoms on the inside of the cyclodextrin molecule, the inside is hydrophobic in comparison with the outside, which contains the secondary and primary hydroxyl groups. The electron density on the inside is relatively high as a result of the inwardly projecting oxygen atoms, so that the cavity represents a soft Lewis base. |
||||||||||||||||||||
 |
||||||||||||||||||||
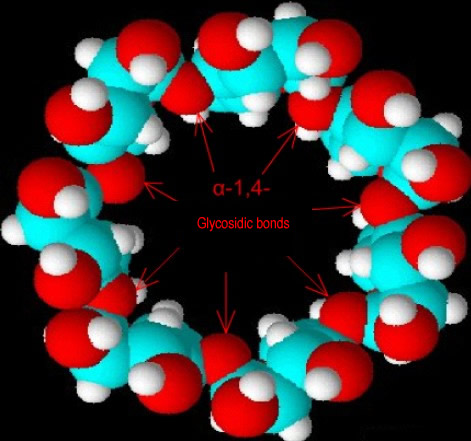
| Fig. 1.17: Bird’s eye view of a β-cyclodextrin molecule; the secondary hydroxyl groups are located on the upper edge | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Table 1.3: Solubility of α-cyclodextrin, β-cyclodextrin and γ-cyclodextrin | ||||||||||||||||||||
|
||||||||||||||||||||
| Table 1.4: Solubility of glucose, fructose, sucrose, β-cyclodextrin and starch | ||||||||||||||||||||
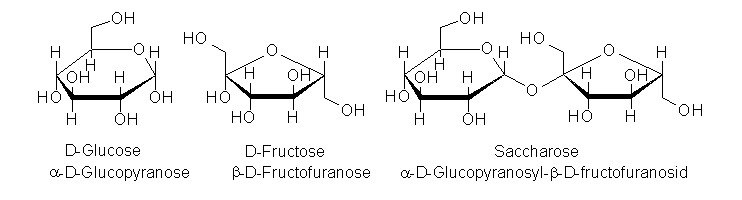
When the solubilities of α-cyclodextrin, β-cyclodextrin and γ-cyclodextrin are compared with each other, significant differences emerge which do not correlate with the ring size. Although α-cyclodextrin and γ-cyclodextrin have similar solubility, β cyclodextrin is approximately ten times less soluble. These differences can be explained by the molecular structure. Each molecule of the readily soluble glucose (see Fig. 1.18) contains five hydroxyl groups, which are able to form hydrogen bonds with the solvent, water. Even the sucrose (saccharose) molecule (see Fig. 1.18) still has eight hydroxyl groups, despite the α-1,4-glycosidic bond, which account for the very high solubility of household sugar in water. Added to this is the significantly greater water solubility of fructose compared with glucose. |
||||||||||||||||||||
 |
||||||||||||||||||||
| Fig. 1.18: Molecular structure of various carbohydrates | ||||||||||||||||||||
 |
||||||||||||||||||||
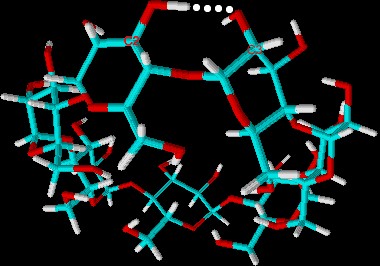
| Fig. 1.19: Intramolecular hydrogen bonds in the β-cyclodextrin molecule | ||||||||||||||||||||
The strongest hydrogen bonds must be formed in β-cyclodextrin, because this molecule is particularly rigid. It is possible to prove that this intramolecular interaction is actually responsible for the low water solubility by derivatizing some of the hydroxyl groups in the cyclodextrin molecule. Methylation yields the cyclodextrin derivative methyl-β-cyclodextrin (see Fig. 1.20), 800 g/l of which can be dissolved in water at 25° C. Solubility in water improves according to the degree of substitution of methoxyl groups for hydroxyl, but decreases again when a certain degree of substitution is reached, since at this point only a few hydroxyl groups remain to form hydrogen bonds. |
||||||||||||||||||||
 |
||||||||||||||||||||
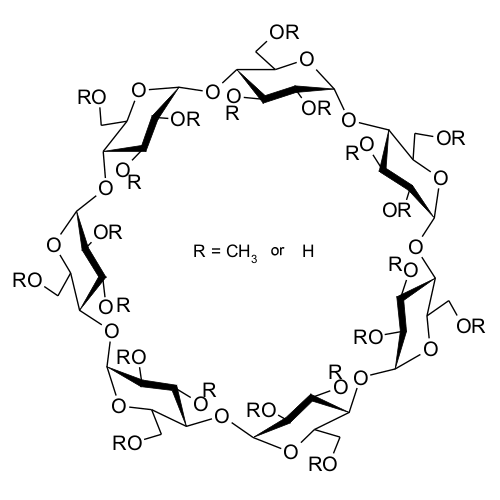
| Fig. 1.20: Molecular structure of methyl β-cyclodextrin | ||||||||||||||||||||
Methylation produces further changes in properties, in addition to considerably increased water solubility. Whilst natural cyclodextrins are sparingly soluble in organic solvents due to the polar molecular exterior, methylation of the hydroxyl groups produces good solubility in organic solvents. The twisting of one glucose unit in the α-cyclodextrin molecule prevents it from forming a regular hexagon. Consequently the intramolecular hydrogen bonds described above are not able to form fully, a fact which leads to improved water solubility relative to β-cyclodextrin. γ-Cyclodextrin is a less rigid molecule due to the greater number of glucose units, which also leads to improved solubility compared with β-cyclodextrin. |
||||||||||||||||||||
References:
|
||||||||||||||||||||
| | Home | Wuppertal University | WACKER | Didactic Dept. | Supp. Info | Experiments | Media | Contact | |