Due to the large number of hydroxyl groups in the cyclodextrin molecule (18, 21 and 24 hydroxyl groups in α-cyclodextrin, β-cyclodextrin and γ-cyclodextrin respectively), the number of possible derivatives of natural cyclodextrins is very high. The hydroxyl groups can be converted to different functional groups in a variety of ways. Therefore it is not especially surprising that in 2003, 1500 derivatives were already known from publications. A vast number of the known derivatives are destined to remain permanent objects of research, since it is too complicated and labor-intensive to synthesize them industrially. |
||||||||
 |
||||||||
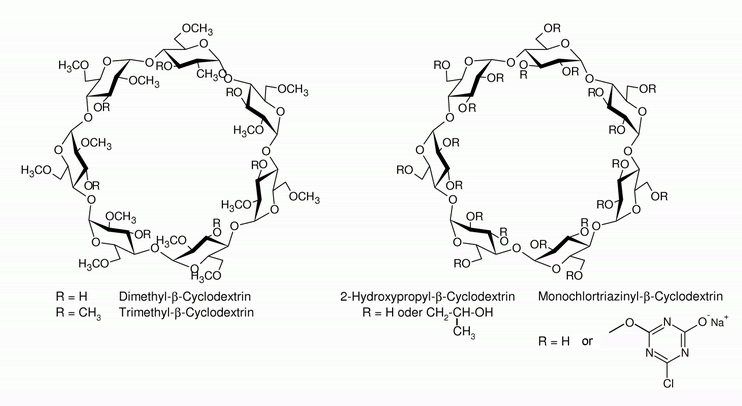
| Fig. 1.28: Molecular structures of the cyclodextrin derivatives manufactured by Wacker Chemie AG | ||||||||
|
||||||||
 |
||||||||
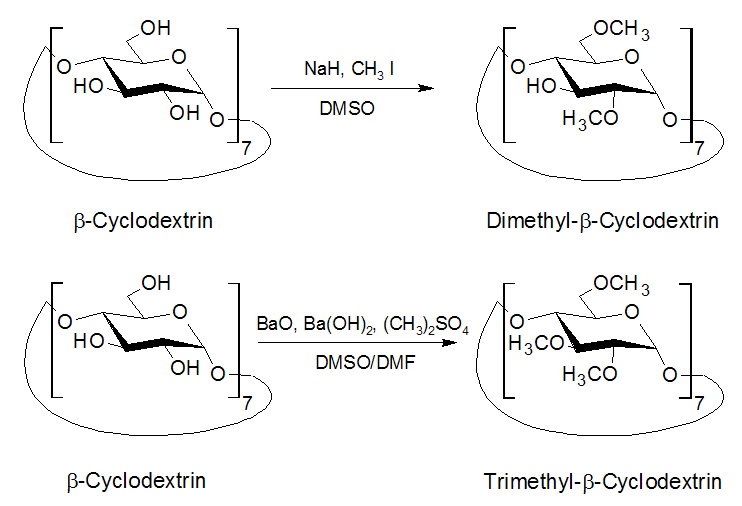
| Fig. 1.29: Different reaction conditions yield tri- or dimethylated cyclodextrin | ||||||||
Partially methylated cyclodextrins can be synthesized due to the different reactivities of the hydroxyl groups at carbon atoms 2, 3 and 6. The hydroxyl group at carbon atom 2 is the most reactive, whilst that at carbon atom 3 is the least reactive. However, in extreme reaction conditions the differences are too slight to allow selective methylation. Derivatization principally modifies water solubility, in addition to the height and the diameter of the lower opening of the cyclodextrin molecule. The introduction of the methyl groups increases water solubility until 2/3rds of the hydroxyl groups have been substituted. If the methylation continues, the product then becomes less water-soluble again. However, even trimethyl-β-cyclodextrin is more soluble than natural β-cyclodextrin. |
||||||||
|
||||||||
| Table 1.7: Solubility of β-cyclodextrin, dimethyl-β-cyclodextrin and trimethyl β cyclodextrin in water | ||||||||
|
||||||||
 |
||||||||
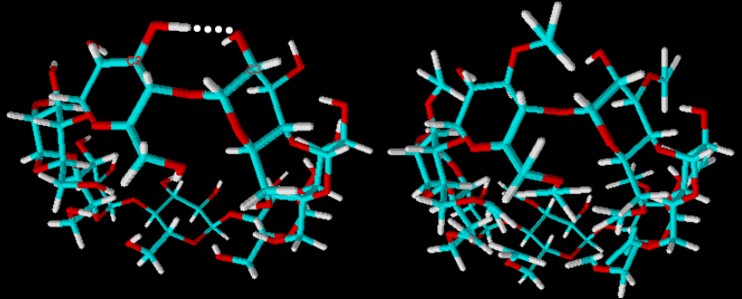
| Fig. 1.30: Comparison of the molecular structure of β-cyclodextrin and dimethyl-β-cyclodextrin | ||||||||
The reason for the improved water solubility of the fully methylated β-cyclodextrin compared with natural β-cyclodextrin cannot be fully explained. The exterior actually becomes considerably more hydrophobic due to the substitution of all hydroxyl groups. But the methyl groups, which are bulkier than the hydrogen atoms, cause steric hindrance on the upper edge of the cyclodextrin molecule that is reduced by tilting the glucose units. In addition, twisting occurs in individual glucose units. These factors lead to increased solubility in water. Derivatization affects not only solubility in water but also solubility in organic solvents. Methylated derivatives are highly soluble in methanol, ethanol and dimethyl sulfoxide due to the hydrophobic exterior, whilst natural β-cyclodextrin is virtually insoluble in these solvents. |
||||||||
References:
|
||||||||
| | Home | Wuppertal University | WACKER | Didactic Dept. | Supp. Info | Experiments | Media | Contact | |