|
4. Solubility |
||||||||||||
As β-cyclodextrin is poorly soluble in water, allow at least 10 minutes for this experiment. |
||||||||||||
Pour 50 ml distilled water into a beaker and weigh out 1 g β-cyclodextrin. Then dissolve small portions of β-cyclodextrin in the water by stirring. Continue to add small portions of β-cyclodextrin to the beaker until no more will dissolve. Then re-weigh the remaining portion of β-cyclodextrin and calculate the solubility at room temperature from the difference. Repeat this experiment with starch. Repeat the experiment with household sugar or glucose to demonstrate the difference in solubility between β-cyclodextrin and starch compared with household sugar or glucose. As mono- and disaccharides are far more soluble, it would take much longer to determine the solubility of glucose or saccharose. For this reason the very considerable differences in solubility are demonstrated only qualitatively, by dissolving a much greater quantity of glucose or saccharose in the same quantity of water. After 0.5 g β-cyclodextrin has been added, the solution turns cloudy. If more β-cyclodextrin is added to the solution, the solid no longer fully dissolves. Even with starch, the addition of a small material portion produces a suspension. In comparison, significantly more glucose or household sugar can be dissolved in the same quantity of water. |
||||||||||||
According to the manufacturer's instructions, the solubility of β-cyclodextrin is 1.85 g /100 ml water at 25° C. The solubility calculated in this experiment is significantly lower at 1 g /100 ml water. Due to the low solubility, the solution would presumably have to be stirred for much longer. The more impressive result in this experiment, however, is the clear difference in the solubility of β-cyclodextrin and starch compared with glucose and saccharose. |
||||||||||||
|
||||||||||||
| Tab. 2.1: Solubility of glucose, fructose, saccharose, β-cyclodextrin and starch | ||||||||||||
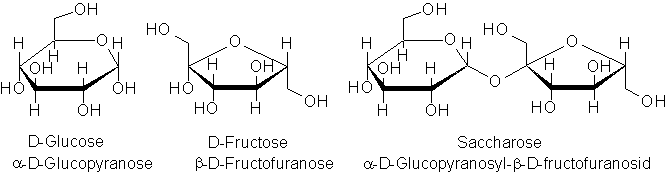
The molecular structure can help to explain these differences. Each glucose molecule possesses five hydroxyl groups, which form hydrogen bonds with the solvent water. The saccharose molecule also has several hydroxyl groups, despite having an α-1,2-glycosidic bond, which accounts for the extremely high water solubility of household sugar. Since fructose is significantly more water-soluble than glucose, sucrose is also more soluble than glucose. |
||||||||||||
 |
||||||||||||
| Fig. 2.7: Molecular structure of different carbohydrates | ||||||||||||
| In the cyclodextrin molecule, as in the macromolecule starch, the α-1,4-glycosidic bonds ensure that only three of the five hydroxyl groups per glucose unit are available to form hydrogen bonds. This explains the low water solubility compared with mono- and disaccharides. | ||||||||||||
To demonstrate the difference in solubility alone, conduct the experiment without the quantification aspect. The experiment clearly shows the relationship between structure and characteristic. In a follow-up lesson you could look at the solubility of the three cyclodextrins with six, seventh and eight glucose units and selected cyclodextrin derivatives. |
||||||||||||
| When the solubility of α-, β- and γ-cyclodextrin are compared initially, clear differences in the solubility emerge, which do not correlate with the ring size. | ||||||||||||
|
||||||||||||
| Tab. 2.2: Solubility of α-, β- and γ-cyclodextrin | ||||||||||||
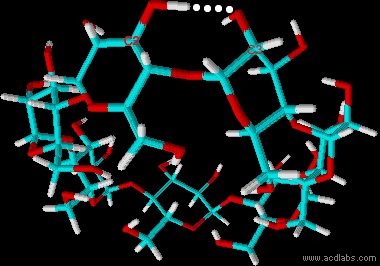
| Whilst α-cyclodextrin and γ-cyclodextrin show similar solubilities, β-cyclodextrin is approximately 10 times less soluble. The smaller number of hydroxyl groups per glucose unit partially explains the reduced solubility of cyclodextrins compared with glucose or saccharose. However, the solubility of β-cyclodextrin is even further reduced by the formation of an intramolecular hydrogen bond between the hydroxyl group on carbon atom 2 and the hydroxyl group on carbon atom 3 of the adjacent glucose unit. | ||||||||||||
 |
||||||||||||
| Fig. 2.8: Intramolecular hydrogen bonds in the β-cyclodextrin molecule | ||||||||||||
| The intramolecular hydrogen bridges are not able to form fully in the α-cyclodextrin molecule, because the contortion of one glucose units prevents the formation of a regular hexagon. This results in improved water solubility compared with β-cyclodextrin. γ-Cyclodextrin is a less rigid molecule due to the greater number of glucose units, and it is therefore more soluble than β-cyclodextrin. |
||||||||||||
|
||||||||||||