  1. What Are Cyclodextrins? 1. What Are Cyclodextrins? |
 |
Cyclodextrins are cyclic oligosaccharides that are formed when starch is degraded by special enzymes known as CGTases. The best-known cyclodextrins are the cyclic oligosaccharides comprising six, seven or eight glucose units, which, like starch, are joined together by α-1,4-glycosidic bonds.
The original name “cellulosine” was coined by A. Villiers, who obtained a crystalline substance in 1891 which reminded him of cellulose due to its resistance to hydrolysis and its non-reducing properties. |
 |
| Fig. 1.9: β-Cyclodextrin is a white powder |
 |
  The microbiologist F. Schardinger made similar observations. He conducted extensive basic research on these substances, which were initially referred to as “Schardinger dextrins.” The cyclic structure would not be postulated until 1936. Today, α-cyclodextrin, β-cyclodextrin and γ-cyclodextrin are the standard names for cyclodextrins containing six, seven and eight glucose units respectively. The less common cyclodextrins containing more than eight glucose units in the molecule are similarly referred to as δ-cyclodextrin and so on. The microbiologist F. Schardinger made similar observations. He conducted extensive basic research on these substances, which were initially referred to as “Schardinger dextrins.” The cyclic structure would not be postulated until 1936. Today, α-cyclodextrin, β-cyclodextrin and γ-cyclodextrin are the standard names for cyclodextrins containing six, seven and eight glucose units respectively. The less common cyclodextrins containing more than eight glucose units in the molecule are similarly referred to as δ-cyclodextrin and so on. |
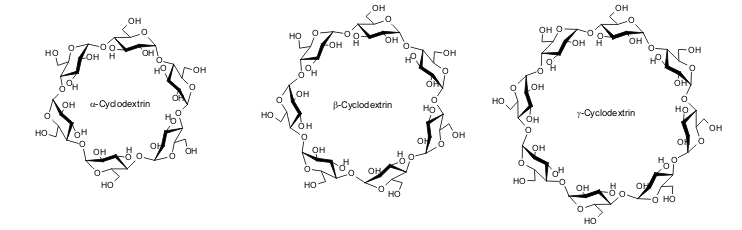 |
| Fig. 1.10: Molecular structure of the natural cyclodextrins |
 |
| Nomenclature based on the names of the structural units is also common (see Table 1.1). |
 |
α-cyclodextrin |
β-cyclodextrin |
γ-cyclodextrin |
Schardinger's α-Dextrin |
Schardinger's β-Dextrin |
Schardinger's γ-Dextrin |
Cyclomaltohexose |
Cyclomaltoheptose |
Cyclomaltooctose |
Cyclohexaglucan |
Cycloheptaglucan |
Cyclooctaglucan |
Cyclohexaamylose |
Cycloheptaamylose |
Cyclooctaamylose |
|
 |
| Table 1.1: Various names for cyclodextrins |
 |
  The numerous hydroxyl groups (18, 21 and 24 respectively in α-cyclodextrin, β cyclodextrin and γ-cyclodextrin) in the cyclodextrin molecules can be derivatized in various ways, and more than 1500 derivatives have been synthesized. The keen interest in cyclodextrins is due to their spatial structure, which resembles an open truncated cone. The cavity within the cyclodextrin molecule can act as a host to different guest molecules. Cyclodextrins have found a wide range of applications due to their ability to form these so-called host-guest complexes. The numerous hydroxyl groups (18, 21 and 24 respectively in α-cyclodextrin, β cyclodextrin and γ-cyclodextrin) in the cyclodextrin molecules can be derivatized in various ways, and more than 1500 derivatives have been synthesized. The keen interest in cyclodextrins is due to their spatial structure, which resembles an open truncated cone. The cavity within the cyclodextrin molecule can act as a host to different guest molecules. Cyclodextrins have found a wide range of applications due to their ability to form these so-called host-guest complexes. |
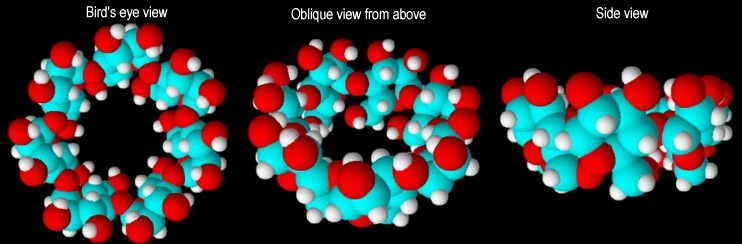 |
| Fig. 1.11: Three-dimensional representation of β-cyclodextrin |
 |
| Animations of different models enable the spatial structure to be studied in more detail. |
 |
References:
- Szejtli, J.; Osa, T.; Comprehensive Supramolecular Chemistry. Volume 3 Cyclodextrins, Elsevier Science Ltd. Oxford, 1996, S. 1, S. 6
- Szejtli, J.; Pure Appl. Chem., 10, 2004, 76, 1825-1845
|
 |
 |
| | Home | Wuppertal University | WACKER | Didactic Dept. | Supp. Info | Experiments | Media | Contact | |


