| The degradation of starch by enzymes gives rise to dextrin, a mixture of polysaccharides of low molecular mass, then to oligosaccharides with fewer glucose units and finally to glucose. The enzyme α-amylase, which is also found in human saliva, is responsible for this degradation. Cyclodextrins, which are cyclic oligosaccharides, are also formed by the enzymatic degradation of starch, but in this case the enzymes belong to the family known as cyclodextrin glycosyl transferases (CGTases). Bacterial strains such as Bacillus macerans, Klebsiella oxytoca and Bacillus circulans produce cyclodextrin glycosyl transferases. |
 |
| Fig. 1.1: Corn is the raw material that provides the starch for cyclodextrin production |
| |
| The industrial production of cyclodextrins requires large quantities of corn starch in addition to the enzymes isolated from the bacteria. Wacker Chemie AG, a globally active company, has been producing cyclodextrins since the 1980s. WACKER’s latest production plant in Eddyville, Iowa (USA) has been manufacturing cyclodextrins since 1999. The proximity of the cornfields was a decisive factor in choosing this production site, where more than 4,000 tons of cyclodextrins are produced annually. |
 |
| Fig. 1.2: The WACKER production plant in Eddyville (USA) is directly adjacent to the cornfields. |
| |
| CGTases are used to break the starch molecules down into segments. Due to the helical structure of the starch, the segments formed consist mostly of seven and, less commonly, six or eight glucose units, which then react further to form cyclic oligosaccharides in a CGTase-typical reaction. The material mixture obtained consists of the three natural cyclodextrins; α-cyclodextrin, β-cyclodextrin and γ-cyclodextrin with six, seven and eight glucose units per molecule respectively. |
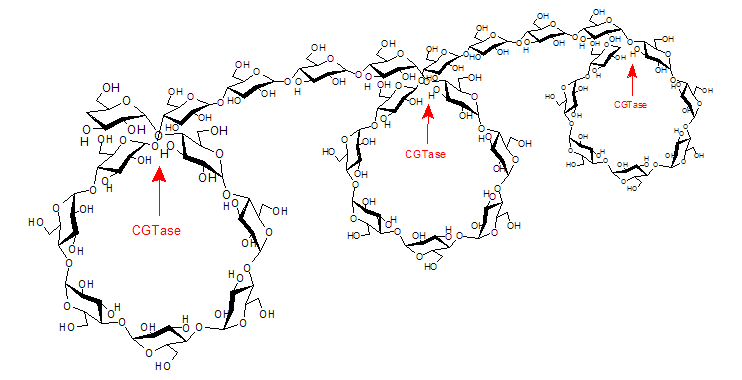 |
| Fig. 1.3: The helical structure of the starch yields mainly cyclodextrins with 6 – 8 glucose units |
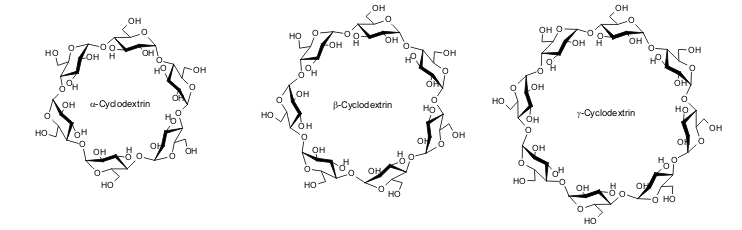 |
| Fig. 1.4: Molecular structure of the natural cyclodextrins |
| Before more was known about cyclodextrin glycosyl transferases, the production of a cyclodextrin was associated with considerable cost, as the resultant material mixture had to be separated. When the enzyme produced by the bacterial strain Bacillus macerans is used, a mixture of the three cyclodextrins with at least 51.2% β-cyclodextrin is obtained. β-Cyclodextrin, containing seven glucose units, is produced in greater yield than α-cyclodextrin and γ-cyclodextrin for energetic reasons. |
The technical production of cyclodextrins can be divided into four key phases:
The reaction takes place in a 30% starch suspension. Since this starch suspension is highly viscous and the cyclodextrin yield would be low, the suspension is initially treated with α amylase, which partially hydrolyzes the starch. The duration of this treatment is optimized to obtain the greatest possible yield of cyclodextrins. The partial degradation of the macromolecules of starch alters its solubility and viscosity. When the optimum degree of starch hydrolysis has been reached, the hydrolysis is terminated by heating, which thermally decomposes the enzyme α-amylase. |
 |
| Fig. 1.5: Stages in cyclodextrin production |
Cyclodextrin glycosyl transferase is added in the next production stage (see Fig. 1.5). The ability of cyclodextrins to form “host-guest complexes” is exploited to significantly increase the yield of cyclodextrin. The cyclodextrin molecules have a cavity that enables them to form complexes, primarily with organic molecules. Since the three cyclodextrins have different-sized cavities , each cyclodextrin has its own range of organic reagents with which it will form a sparingly soluble (product) complex and precipitate out of solution. The result of such precipitation is continuous removal of cyclodextrin product from the system. The system tries to restore the equilibrium by forming more of the product and so the cyclodextrin yield increases significantly. The fact that a solid is formed is an additional advantage because the product can be separated from the residual starch solution by filtration. The solid complex is washed and then re-suspended in water. The organic molecules that have been complexed in the cyclodextrin cavities are removed again by steam distillation or by extraction with a suitable organic solvent. Further cleanup stages then follow (see Fig. 1.5). |
 |
| Fig. 1.6: Complex formation during cyclodextrin production is used to increase the yield |
For an organic compound to be a suitable complexing agent, it must form a sparingly soluble complex with only one of the three cyclodextrins. However, that is not the only determining factor in the choice of complexing reagents. The production of pure cyclodextrins requires that the complex can be totally dissociated by steam distillation or extraction. 1-Decanol is the complexing agent of choice for α cyclodextrin, the smallest of the three cyclodextrins, whilst toluene is used for β cyclodextrin. The much larger cyclohexadec-8-en-1-one is used for γ-cyclodextrin. |
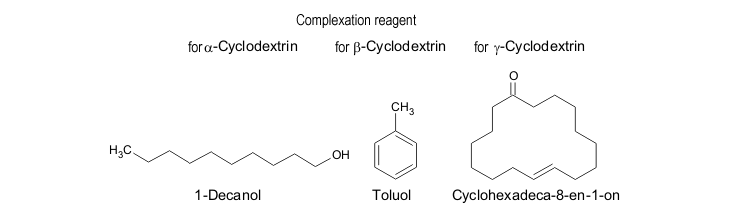 |
| Fig. 1.7: Complexing reagents for the precipitation of a cyclodextrin as a solid complex |
| For a long time, complexation was the only way to increase the cyclodextrin yield, because the enzyme catalyzes the formation of all three cyclodextrins. However, the elucidation of the DNA sequence in the CGTases has made it possible to isolate selective CGTases (α-CGTase, β-CGTase and γ-CGTase), which further increases the yield. Combined use of the appropriate CGTase and the right complexing agent now enables all three natural cyclodextrins to be obtained in a yield of virtually 100%. |
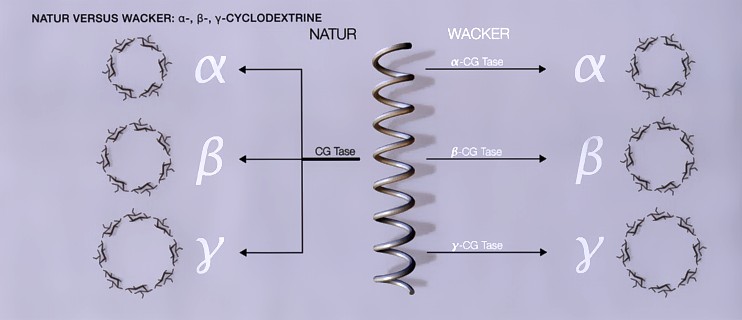 |
| Fig. 1.8: Specific enzymes facilitate the production of pure cyclodextrins |
References:
|
| | Home | Wuppertal University | WACKER | Didactic Dept. | Supp. Info | Experiments | Media | Contact | |