 |
5. Complexation of Iodine and Detection of Complex |
 |
  1
Materials, Chemicals, Time Needed 1
Materials, Chemicals, Time Needed |
 |
- Scales
- Spatula
- Glass beaker (150 mL)
- Hot plate with magnetic stirrer
- Magnetic stir bar
- Measuring cylinder
- Thermometer
- Suction filtration flask with rubber seal
- Büchner funnel
- Filter paper
- Water jet pump
- Test tubes
- Test tube stands
- Bunsen burner
- Iodine Xn, N
- Potassium iodide
- β-Cyclodextrin
- Distilled water
- Heptane F, Xn, N
- Starch solution
Allow approximately 20 minutes to make the complex. After drying, the detection reactions take only a few minutes. |
 |
  2
Procedure and Observations 2
Procedure and Observations |
 |
Prepare a solution of 0.25 g iodine, 1.65 g potassium iodide and 60 ml water. Treat the solution with 0.5 g β-cyclodextrin and stir for 10 minutes at 80°C. Make sure that all the iodine has dissolved. Cool the solution to room temperature and obtain the precipitate by suction. Dry the solid matter and then perform the following tests in a test tube:
a) Add several milliliters of starch solution to a spatula tip of solid and shake.
b) Treat a spatula tip of solid with heptane and shake.
c) Heat a small portion of the solid in a test tube with a Bunsen burner. Then add a little starch solution.
d) Add water to a spatula tip of the solid and shake vigorously. Then cover the aqueous solution with heptane and wait little while before shaking again.
Check the observations after a few minutes.
A precipitate forms from the dark brown solution during cooling. Dark brown, needle-shaped crystals are discernible during suction filtration. |
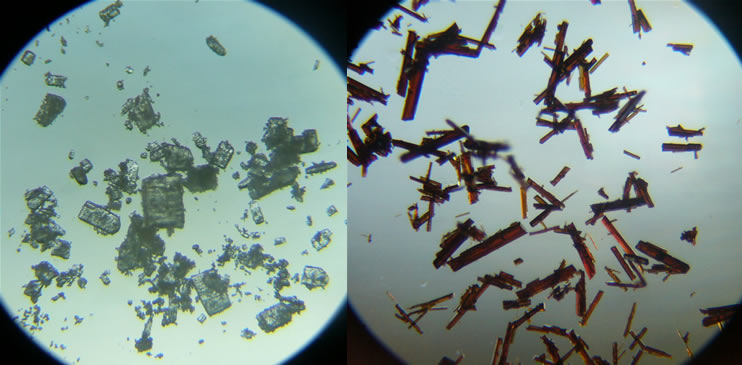 |
Fig. 2.9: Crystals of β-cyclodextrin and the iodine-β-cyclodextrin complex under the microscope (x4 magnification) |
 |
a) The crystals do not visibly dissolve. The starch solution remains colorless. The solution gradually turns blue after approximately 5 minutes.
b) There is no discernible discoloration of the heptane phase initially, but it gradually turns very faintly violet (much such less intense than in d)).
c) Violet vapors rise in the test tube during heating. When the starch solution is added, the solution immediately turns dark blue.
d) Initially the heptane phase remains colorless. Only after some time does the heptane phase turns violet after shaking. |
 |
Complex treated with starch solutiont |
Complex treated with heptane |
Complex heated and treated with starch solution |
Complex treated with water and heptane |
|
 |
| Fig. 2.10: Detection reactions immediately after adding the reagents |
 |
 |
Detection of iodine with starch solution |
No discoloration of the heptane phase |
Detection of iodine with the starch solution |
Detection of iodine by discoloration of the heptane phase |
|
| Fig. 2.11: Detection reactions after eight minutes |
 |
|
  3
Discussion of Results 3
Discussion of Results
|
 |
The negative result of the iodine-starch reaction in the first test tube indicates that the solid does not contain free iodine. The crystals are a host-guest complex in which iodine molecules are encapsulated in the cavity of the cyclodextrin molecule. The complex is stabilized by Van der Waals forces acting between the non-polar iodine molecules and the hydrophobic interior of the β-cyclodextrin molecule. However, the forces driving complexation can be attributed to other factors. One key factor is that water molecules present in the cyclodextrin cavity are substituted by the guest molecule. |
 |
|
α-Cyclodextrin |
β-Cyclodextrin |
γ-Cyclodextrin |
Hydrate formation |
α-CD * 6 H2O |
β-CD * 11 H2O |
γ-CD * 15.7 H2O |
Number of water molecules in the cavity |
1 – 2 |
6 |
8.8 |
|
 |
| Tab. 2.3: Hydration of cyclodextrins |
 |
These water molecules have a relatively high enthalpy, since there are no interactions between the polar water molecules and the non-polar interior of the cyclodextrin molecule. The substitution of the water molecules with a less polar guest molecule releases energy. The increase in entropy favors this process. In addition, van der Waals forces to the non-polar iodine molecules are formed in the cyclodextrin cavity.
Dissociation of the iodine-β-cyclodextrin complex can be achieved by heating the solid. The vapor color and the positive result of the iodine-starch reaction indicates that the iodine has been expelled. Gradual dissociation can also be achieved by adding water. The concentration difference between the guest in the complex and in the solution results in the dissociation of the complex until equilibrium is reached. The formation of the blue inclusion compound and the color of the heptane phase indicate that the complex dissociates after some time.
Dissociation of the complex is barely discernible during the addition of heptane. It could be assumed that the heptane molecules would replace the iodine molecules as the guest component, but this is not shown in the experiment. |
 |
  4
Tips and Comments 4
Tips and Comments
|
 |
| To get clear results, the complexes produced from the solution must be dry initially, since otherwise the iodine-starch reaction would give a positive result immediately after the starch solution were added. This is because water triggers complex dissociation, as the experiment shows. For this reason the experiment cannot be completed in one lesson. |
 |
  5 Supplementary Information 5 Supplementary Information |
 |
This experiment demonstrates the complexation of iodine by the absence of typical reactions of the guest molecule, for example the highly sensitive iodine-starch reaction. This method is also used in the experiment on the complexation of salicylic acid.
Spectroscopic methods are used to study host-guest complex formation with different guest molecules. NMR (nuclear magnetic resonance) investigations provide direct evidence of complex formation. This is achieved by comparing 1H-NMR spectra of cyclodextrin, the guest molecule and the complex in D2O.The signals of the cyclodextrin-hydrogen atoms are displaced in comparison with the signals in the spectrum for pure cyclodextrin as a result of interactions between the guest molecule and the cyclodextrin molecule. Since only the hydrogen atoms present in the cavity of the cyclodextrin molecule at carbon atoms 3 and 5 (see Fig. 2.12) undergo a chemical shift, the spectra can be rapidly interpreted and evaluated. The hydrogen atoms on the exterior of the cyclodextrin molecule are not affected by the complexation of a guest molecule.
|
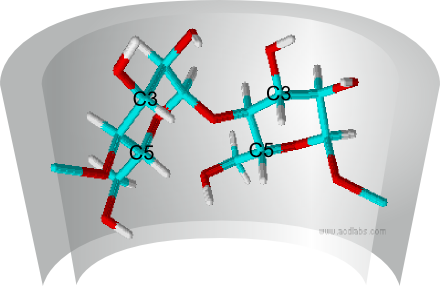 |
| Fig. 2.12: View of two glucose units in the β-cyclodextrin showing the inward-facing hydrogen atoms at carbon atoms 3 and 5 |
 |
| If the guest molecules are colored substances, UV-VIS spectroscopy can be used to indicate the formation of a host-guest complex, since the absorption spectrum of the guest molecule changes. |
 |
  6 References 6 References |
 |
- Szejtli, J.; Pure Appl. Chem., 10, 2004, 76, 1825-1845
- Connors, K. A.; Chem. Rev., 1997, 97, 1325-1357
- Szejtli, J.; Osa, T.; Comprehensive Supramolecular Chemistry. Volume 3 Cyclodextrins, Elsevier Science Ltd. Oxford, 1996, S. 21-22
- Szejtli, J.; Osa, T.; Comprehensive Supramolecular Chemistry. Volume 3 Cyclodextrins, Elsevier Science Ltd. Oxford, 1996, S. 200
|
 |
  | Home | Wuppertal University | WACKER | Didactic Dept. | Supp. Info | Experiments | Media | Contact | | Home | Wuppertal University | WACKER | Didactic Dept. | Supp. Info | Experiments | Media | Contact | |



