 |
9. Stabilization of O/W Emulsions |
 |
  1
Materials, Chemicals, Time Needed 1
Materials, Chemicals, Time Needed |
 |
- Two 100 mL measuring cylinders with bungs
- Test tubes
- Test tube stands
- Distilled water
- Cooking oil
Allow 5 – 10 minutes for the first part of this experiment. Testing the different types of emulsion takes a further 5 minutes. |
 |
  2
Procedure and Observations 2
Procedure and Observations |
 |
Add 20 ml of water to each measuring cylinder and cover with 20 ml oil. Add 0.25 g β-cyclodextrin to the first cylinder and then shake both cylinders vigorously. Observe the breaking of the emulsions. If the two samples look significantly different, shake the cylinder containing the emulsion with β-cyclodextrin vigorously again and then divide this emulsion between three test tubes. Add some water to the first test tube and some oil to the second. The third sample is a control.
The emulsion without the β-cyclodextrin breaks into two phases of equal volume after just a short time, although both phases remain cloudy. The other emulsion appears to be stabilized, because a thin aqueous phase appears at the bottom of the measuring cylinder after only a few minutes. The emulsion of water, oil and cyclodextrin can be diluted with water, but not with oil. When oil is added, it floats on the surface of the milky emulsion as the second phase, despite gentle shaking. |
 |
|
  3
Discussion of Results 3
Discussion of Results
|
 |
The first part of this experiment demonstrates the effectiveness of β-cyclodextrin as an emulsifier, since the emulsion breaks down considerably more slowly after the addition of cyclodextrin.
The fatty acids in the fat molecules form a host-guest inclusion complex with the cyclodextrin. Unlike surfactants, which co-solubilize water and oil by entrapping tiny fat droplets in micelles, a cyclodextrin molecule forms an inclusion complex with only one or two fat molecules. Several cyclodextrin molecules can also be involved in the complexation of a single fat molecule, depending on the length of the fatty acid alkyl chain.
The fat molecule is encased in a water-soluble shell, which stabilizes the emulsion, since no van der Waals forces can form between the alkyl chains, and the fat molecules can no longer form an agglomeration. |
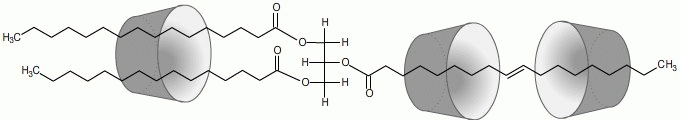 |
| Fig. 2.19: Example of the complexation of a fat molecule by three cyclodextrin molecules |
 |
The aim of the second part of this experiment is to test the type of emulsion. Since the emulsion can be diluted with water, but not with oil, it is an oil-in-water emulsion (O/W). The β-cyclodextrin molecules enclose the non-polar alkyl chains of the fatty acid residues in the fat molecules like pearls threaded on a string. The water therefore represents the outer, active phase. Since there are more fat molecules than β-cyclodextrin molecules and complexation of the alkyl chains is not quantitative, the emulsion cannot be fully stabilized. |
 |
  4
Tips and Comments 4
Tips and Comments
|
 |
  5 Supplementary Information 5 Supplementary Information |
 |
The emulsifying action of cyclodextrins is used to convert lipophilic substances to a water-soluble form. For example, water-insoluble β-carotin is converted to a water soluble form in a cyclodextrin complex.
In addition to increasing the water-solubility of guest molecules, complexation with cyclodextrins has other benefits, as in the complex of γ-cyclodextrin with omega-3(,6) fatty acids, for instance. These complexes are manufactured by WACKER under the brand name OmegaDry®. Clinical studies suggest that omega-3-fatty acids, especially those extracted from fish oils and algae, are beneficial to health. They can reduce the risk of coronary heart disease, because they have a positive effect on the composition of blood lipid levels. Encapsulation inside cyclodextrins provides the ideal solution, since these oils have a strong characteristic taste and are additionally extremely oxidation-sensitive. Their appearance in the form of a colorless, odorless powder means that they can easily be added to dough in bakery products, for instance.
|
 |
  6 References 6 References |
 |
- Reuscher, H.; Flexible Building Blocks for Healthy Eating; WACKER WORLD WIDE CORPORATE MAGAZINE 2.4, 2004, 23-25
- Szejtli, J.; Osa, T.; Comprehensive Supramolecular Chemistry. Volume 3 Cyclodextrins, Elsevier Science Ltd. Oxford, 1996, S. 317, S. 330-332
- Tausch, M.; von Wachtendonk, M.; Chemie 2000+ Band 3, C.C. Buchners Verlag Bamberg, 2005, S. 81, S. 95
- WACKER FINE CHEMICALS; CAVAMAX® OmegaDry® Cyclodextrins in food and nutraceutical applications
- Reuscher, H.; Flexible Building Blocks for Healthy Eating; WACKER WORLD WIDE CORPORATE MAGAZINE 2.4, 2004, 23-25
|
 |
  | Home | Wuppertal University | WACKER | Didactic Dept. | Supp. Info | Experiments | Media | Contact | | Home | Wuppertal University | WACKER | Didactic Dept. | Supp. Info | Experiments | Media | Contact | |
|
