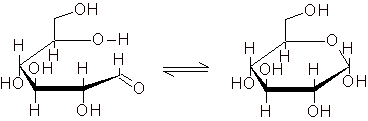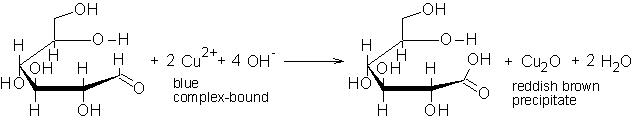|
3. Hydrolysis of β-Cyclodextrin and Detection of the Degradation Product |
 |
  1
Materials, Chemicals, Time Needed 1
Materials, Chemicals, Time Needed |
 |
- Test tubes
- Test tube stands
- Test tube clamps
- Bunsen burner
- Spatula
- Scales
- pH indicator paper
- Boiling stone
- β-Cyclodextrin
- Glucose
- Distilled water
- Concentrated hydrochloric acid C
- Sodium hydroxide solution, c = 2 mol/L C
- Sodium carbonate Xi
- Fehling’s solution I Xn
- Fehling’s solution II C
Allow 20 minutes to prepare the samples and perform the hydrolysis. Neutralization and the Fehling’s test with the three sample solutions takes a further 10 minutes. |
 |
  2
Procedure and Observations 2
Procedure and Observations |
 |
First, dissolve 0.2 g β-cyclodextrin in 20 ml water and then divide the solution between two test tubes. To the first test tube, add 3 ml concentrated hydrochloric acid and heat the solution. Boil for 10 minutes and then allow to cool to room temperature. Neutralize the solution by adding portions of sodium carbonate (test the pH with indicator paper). Then add approx. 4 ml Fehling’s solution (Fehling I and II in the same proportions) and heat gently. Repeat the Fehling’s test on the β-cyclodextrin solution without hydrochloric acid (in the second test-tube) and on a solution of 0.1 g glucose in 10 ml water.
The Fehling’s test is positive in the case of the glucose solution and the β-cyclodextrin solution treated with hydrochloric acid, whereas the pure β-cyclodextrin solution does not change color after addition of the Fehling’s solution and heating. |
 |
| Fig. 2.3: The Fehling's test results on the β-cyclodextrin solution before and after hydrolysis, and on the glucose solution |
 |
The test can also be performed under alkaline conditions for comparison. Cyclodextrins, like other polysaccharides, are considerably more stable to hydrolysis under alkaline conditions, so the result of the Fehling’s test is negative even after prolonged boiling of an alkaline solution of β-cyclodextrin with sodium hydroxide solution c = 2 mol/l. |
 |
|
  3
Discussion of Results 3
Discussion of Results
|
 |
The negative result of the Fehling’s test on the β-cyclodextrin solution shows that it possesses no aldehyde groups. No equilibrium can exist between the chain and ring structure of the glucose building units due to the α-1,4-glycosidic bonding of the glucose building units (see Fig. 2.5). The α-1,4-glycosidic bonds break down during acid hydrolysis of the β-cyclodextrin. |
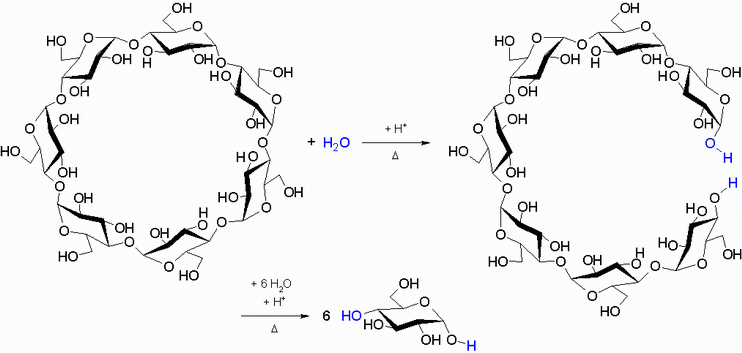 |
Fig. 2.4: Hydrolysis of the α-1,4-glycosidic bond in β-cyclodextrin |
 |
Oligosaccharides form initially, and then break down further to form glucose due to acid-catalyzed hydrolysis. In addition to the glucose molecules, the ring-opened β-cyclodextrin molecules (see Fig. 2.4) and the other degradation products (oligosaccharides with 6 to 2 glucose units) are subject to the equilibrium between the chain structure and the ring structure (see Fig. 2.5). |
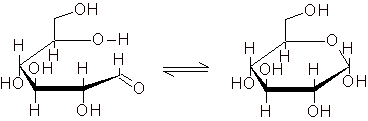 |
Fig. 2.5: Equilibrium between the chain and ring structure of the glucose molecule |
 |
The balanced chain structure possesses an aldehyde group, which accounts for the positive Fehling's test. |
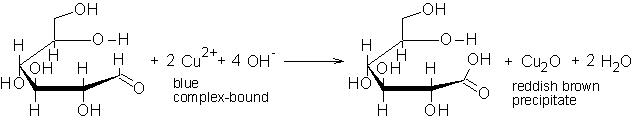 |
Fig. 2.6: Reaction equation of the Fehling's test with glucose |
 |
  4
Tips and Comments 4
Tips and Comments
|
 |
This experiment can also be performed on hot plates in glass beakers. If glucose test strips are available, you can save time by omitting the Fehling's test. |
 |
  5 Supplementary Information 5 Supplementary Information |
 |
Compared with β-cyclodextrin, α-cyclodextrin is considerably more resistant to hydrolysis in acid solutions. Even after three hours in extremely acidic conditions (pH = 2.4) at 100° C, no breakdown of α-cyclodextrin is discernible.
In the case of β-cyclodextrin, acid hydrolysis is initiated by the protonation of a glycosidic oxygen atom and continued by the cleavage of the glycosidic bond. The resistance to hydrolysis of cyclodextrins in alkaline conditions can be explained by the fact that, with hydroxide ions, neither proton extraction from the cyclodextrin molecule nor nucleophilic attack of the carbon atom in the cyclodextrin molecule can occur.
|
 |
  6 References 6 References |
 |
- WACKER Chemical Corporation; CAVAMAX® α-Cyclodextrin – the new soluble dietary fiber
- Reuscher, H.; Flexible Building Blocks for Healthy Eating; WACKER WORLD WIDE CORPORATE MAGAZINE 2.4, 2004, 23-25
- Morrison, R. T.; Boyd, R. N.; Lehrbuch der Organischen Chemie; VCH Verlagsgesellschaft mbH, 1986, S. 611
- Tausch, M.; von Wachtendonk, M.; Chemie 2000+ Band 1, C.C. Buchners Verlag Bamberg, 2001, S. 104-105
|
 |
  | Home | Wuppertal University | WACKER | Didactic Dept. | Supp. Info | Experiments | Media | Contact | | Home | Wuppertal University | WACKER | Didactic Dept. | Supp. Info | Experiments | Media | Contact | |