|
8. Host-Guest Complex with Thermochromic and Photochromic Properties |
||||||||||
A stirring times of six hours is required to make the complex. Once the solid has been obtained by suction filtration and dried, it can be used in the chemistry lesson. The test for thermochromic and photochromic characteristics can be performed in 15 minutes. The chemical spiropyran can be ordered from a chemical supplier or manufactured in a one-step synthesis. |
||||||||||
|
||||||||||
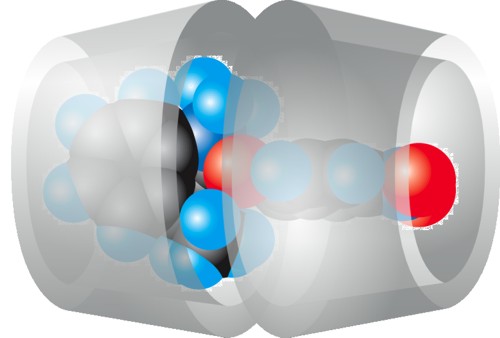
In this experiment a host-guest complex is formed with the largest of the three natural cyclodextrins and spiropyran (see Fig. 2.38). Spiropyran is initially present as a neutral molecule and is not water-soluble due to its molecular structure, so consequently complexation from an aqueous solution is not feasible. For this reason ethanol with a small amount of water is used as the solvent. A complex forms in the suspension during stirring, in which two γ-cyclodextrin molecules encapsulate one spiropyran molecule (see Fig. 2.37). |
||||||||||
 |
||||||||||
| Fig. 2.37: The γ-cyclodextrin complex with spiropyran has the host:guest stoichiometric ratio of 2 : 1 | ||||||||||
In this cyclodextrin complex van der Waals forces stabilize parts of the spiropyran molecule in the cyclodextrin cavities. The right-hand part of the molecule can also form a hydrogen bond with the nitro group to the primary hydroxyl groups on the lower edge of the cyclodextrin molecule. |
||||||||||
 |
||||||||||
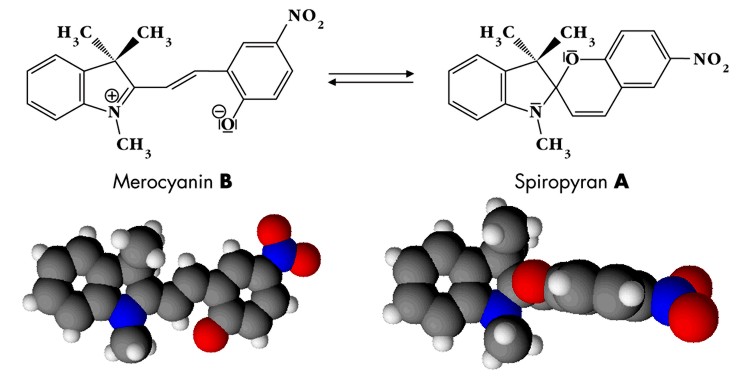
| Fig. 2.38: Molecular structures and three-dimensional models of the isomers merocyanine and spiropyran | ||||||||||
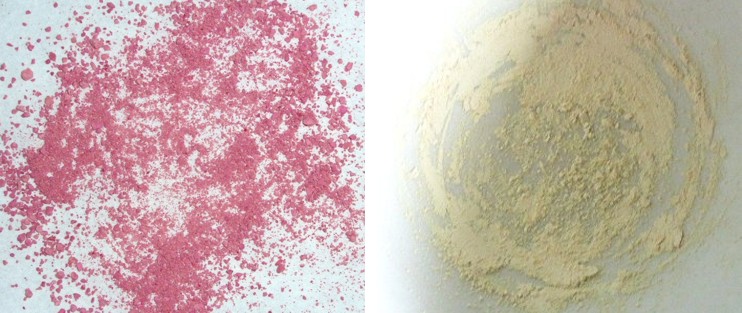
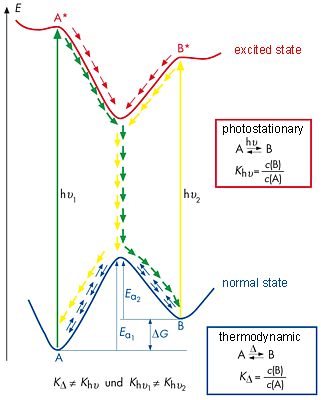
The color changes observed during exposure to light and heat show that isomerization can occur in the cyclodextrin molecules despite complexation. The intermolecular interactions between the cyclodextrin molecules and parts of the guest molecule are ruptured during isomerization and then have to reform, since the spatial structures of merocyanine and spiropyran are very different. The formation of spiropyran from merocyanine creates a spiro center, in which the two parts of the molecule are arranged perpendicular to one another and are rigid (see three-dimensional model in Fig. 2.38), whilst the merocyanine molecule pivots around the single bonds in the center. The system of conjugated double bonds in merocyanine is considerably larger than in spiropyran, so the bluish violet color of the solid can be attributed to the formation of merocyanine. Spiropyran forms when the solid is exposed to visible light. Here, there is no extensive system of conjugated double bonds due to the spiro center. The pink color is discernible when the two isomers are in equilibrium. Fig. 2.39 shows the energy diagram representing the energy levels of the two isomers A and B in the normal state and in the electrically excited states A* and B*. Isomer A, the more thermodynamically stable of the two, is spiropyran, which in the experiment reacts to the violet colored merocyanine (B) by thermal isomerization. The diagram explains why the color reversal to the pink solid occurs quickly. Heating generates sufficient activation energy to re-enter the more thermodynamically stable state A (blue curve in Fig. 2.39). However, this reverse reaction B → A is incomplete, resulting in a mixture of spiropyran and merocyanine. |
||||||||||
 |
||||||||||
| Fig. 2.39: Energy diagram of the spiropyran – merocyanine system | ||||||||||
Photochemical isomerization occurs during light exposure, in which the merocyanine is converted to the excited state by absorption of a suitable photon. This then reacts to form spiropyran (A) (shown in yellow in Fig. 2.39). Since spiropyran is more thermodynamically stable, no reverse reaction takes place, so consequently the yellowish color persists for longer. The equilibrium between the isomers is then strongly on the spiropyran (A) side. |
||||||||||
β-Cyclodextrin cannot form a complex with spiropyran. The solid is colorless. The internal diameter of the β-cyclodextrin molecule is too small for the spiropyran. The complexated spiropyran acts as a molecular switch, which can be converted from one form to the other in the presence of light or heat. The photochromic and thermochromic properties of spiropyran-merocyanine can also be observed when spiropyran is dissolved in toluene or nail polish. |
||||||||||
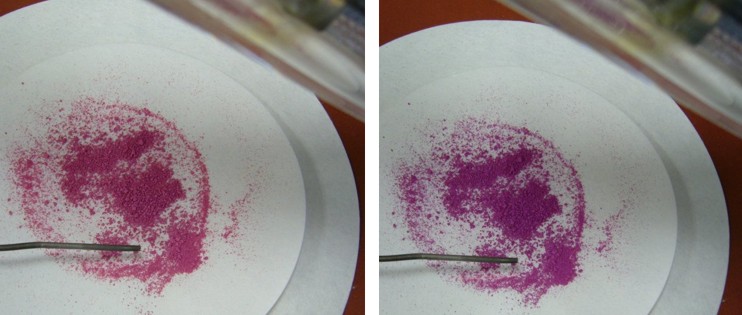
In the experiment the isomerization of spiropyran to merocyanine was achieved only by the thermal route. Merocyanine can also be produced photochemically. This requires a higher energy light source, due to the greater energy difference between the normal state and the excited state of the spiropyran. UV light from a water-cooled 150W high-pressure mercury lamp is required to excite spiropyran. After a while the solid turns violet under this light. To exclude the possibility that thermal isomerization has occurred, the high-pressure mercury lamp is water-cooled and temperature is controlled with a thermal sensor. |
||||||||||
 |
||||||||||
| Fig. 2.40: The photochemical isomerization of spiropyran to merocyanine occurs only with high-energy UV light | ||||||||||
|
||||||||||